Aratana Therapeutics receives FDA approval of canine appetite stimulus
Entyce is a first-of-its-kind therapeutic for inappetent dogs.
Images courtesy of Aratana TherapeuticsAratana Therapeutics Inc. has received approval from the U.S. Food and Drug Administration's Center for Veterinary Medicine (CVM) of Entyce-a first-of-its-kind ghrelin receptor agonist that stimulates appetite in dogs-according to a company release.
Entyce, a new chemical entity, works by mimicking the hunger hormone ghrelin to stimulate appetite. The prescription medication is a flavored liquid that is administered orally.
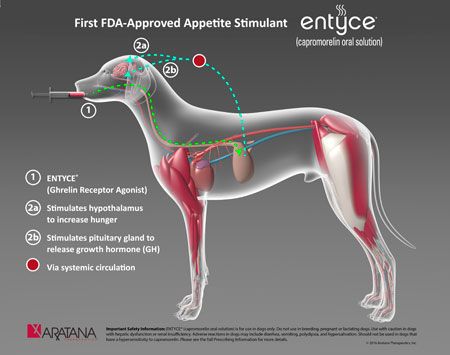
“Nearly 10 million dogs are diagnosed with inappetence each year and we believe Entyce will fulfill a significant unmet need to stimulate appetite in dogs,” says Steven St. Peter, MD, president and chief executive officer of Aratana Therapeutics, in the release. Aratana is reportedly planning a February 2017 commercial launch of Entyce in order to coincide with the North American Veterinary Conference.
FDA approves oral drug for broad canine protection against parasites
October 7th 2024Elanco's lotilaner, moxidectin, praziquantel, and pyrantel chewable tablets (Credelio Quattro) provide a single monthly dose for protection against fleas, ticks, heartworms, roundworms, hookworms, and 3 species of tapeworm.
Read More
Podcast CE: A Surgeon’s Perspective on Current Trends for the Management of Osteoarthritis, Part 1
May 17th 2024David L. Dycus, DVM, MS, CCRP, DACVS joins Adam Christman, DVM, MBA, to discuss a proactive approach to the diagnosis of osteoarthritis and the best tools for general practice.
Listen