Arthrocentesis: Quick cytologic diagnosis of orthopedic conditions (Proceedings)
Synovial fluid analysis is an essential test for diagnosing joint disease in dogs and cats.
Synovial fluid analysis is an essential test for diagnosing joint disease in dogs and cats. It can distinguish inflammatory from non-inflammatory arthropathies, and may help determine the underlying cause of either. Arthrocentesis is a relatively quick and simple procedure to perform. It requires some basic knowledge of joint anatomy, minimal equipment, and can be performed under sedation. It is low risk for the patient, and has the potential for high yield results.
Indications
The workup for joint disease begins with a good history, physical examination, and radiographic imaging. Synovial fluid analysis may be indicated for any dog with joint pain or effusion. Most commonly, we use it to definitively diagnose inflammatory arthritis, either infectious or non-infectious. These animals may present with generalized stiffness ± fever, with pain and effusion in multiple joints, or with a lateralized lameness.
Animals with immune-mediated joint disease typically have multiple joint involvement. Most often, the distal limb joints- carpus and tarsus- are more affected. Rickettsial arthritis can be polyarticular as well. It is important to document range of motion in all joints, as some affected joints may only show a decreased range of motion or pain with extreme flexion or extension. If polyarthritis is suspected, multiple joints should be aspirated. Some authors recommend sampling of a minimum of 6 joints, to help ensure positive findings in at least 2 joints.
Septic arthritis is more commonly monoarticular, and often affects the more proximal joints- stifle, elbow, shoulder, hip. These cases may have severe joint effusion, and sometimes exhibit accompanying cellulitis.
Background
Joints are comprised of two opposing surfaces of articular hyaline cartilage. The joint is supported by a joint capsule (synovial membrane or synovium) and bathed in synovial fluid. Cartilage is composed of a proteoglycan matrix, collagen, and water. Routine cartilage metabolism and maintenance produce degradative enzymes such as MMP-3 and proteoglycan fragments, which can be found in the synovial fluid. Synovial fluid is a highly viscous ultrafiltrate of blood plasma. The synoviocytes of the joint capsule play a critical role in maintaining the composition of this fluid. Type A synoviocytes are mobile resident macrophages of the joint. They are responsible for phagocytosis and antigen-presentation. Type B synoviocytes are fixed in the villi of the synovium, and are primarily secretory. They produce hyaluronic acid, collagen, and fibronectin. Hyaluronic acid provides the viscous property of joint fluid. As a group, synoviocytes compose the synovial membrane, providing selective permeability to molecules in plasma <12kD in size. Larger molecules, such as the plasma protein fibrinogen, are not present in joint fluid. Thus, because of filtration, secretion, and cartilage metabolism, normal joint fluid differs from plasma by the absence of plasma proteins, and the presence of hyaluronate, mononuclear cells, proteoglycans, and inflammatory mediators. In addition to lubricating the joint, synovial fluid enhances joint stability and provides nutrients to articular cartilage.
The pathogenesis of joint effusion is similar regardless of the specific inciting event. Chondrocytes and synoviocytes release cytokines, which cause vasodilation of the synovial capillaries. This vasodilation and the accompanying increased vascular permeability permit fluid, protein, and inflammatory cells to enter the joint. These white blood cells contribute to the inflammatory cascade, causing release of degradative enzymes from additional cells. The type and number of leukocytes determine the clinical features of the arthritis.
Equipment
Arthrocentesis requires
1. Hair clippers
2. Surgical prep- chlorhexidine scrub or betadine scrub
3. Sterile gloves
4. Several 1-1½ " x 22-25 ga needles. The specific length and size will vary based upon the joint being sampled, but should be the smallest size possible to avoid damage to the articular cartilage.
5. 3mL syringe
6. Glass slides
7. EDTA tube (purple top)
8. Culturette
9. Diff-Quick stain
Procedure
Before aspirating, the patient should be adequately sedated. Typically, a combination of butorphanol (0.2mg/kg) and acepromazine (0.01mg/kg) is sufficient. However, with young, healthy dogs and cats (especially fractious ones) I don't hesitate to use a combination of butorphanol (0.2mg/kg) and medetomidine (5-15mcg/kg).
Asepsis is important to avoid causing septic arthritis and for accurate culture results. The joint to be sampled should be clipped and prepared with surgical scrub (chlorhexidine or betadine scrub). Once prepared, the area should be handled only by sterile gloved hands.
Palpate the bony landmarks of the joint and locate the needle insertion point. Attach the needle to the syringe and advance the needle slowly into the joint space. Upon entering the joint, a popping sensation may be felt. Aspirate joint fluid with gentle suction. Before withdrawing the needle, release the syringe plunger, to avoid aspirating blood from a capillary and contaminating the sample. Proper aspiration may yield only small amounts of fluid- it is unusual to obtain more than 0.05 to 0.3mL from a normal joint, and even an inflamed joint may yield little fluid. If no fluid is aspirated, release suction, partially withdraw the needle, and redirect the needle.
Ideally, enough fluid is aspirated to prepare slides for cytology, perform fluid analysis, and culture. If only a small amount of fluid is aspirated, the priority should be to make slides for cytology. Because abnormal joint fluid is more likely to clot, fluid for analysis should be placed in a small EDTA tube. It can be stored for up to 24 hours in the refrigerator. Only a small drop is needed on a culturette for culture. Alternatively, growth medium can be aspirated into the empty syringe, and then expelled back into the sterile container.
Gross evaluation
Synovial fluid can be evaluated grossly for color, turbidity, and viscosity. Normal fluid is colorless. Hemarthrosis appears as a uniformly red tap, while a blood-contaminated tap is inhomogenously red. Yellow-orange tinged fluid is an indicator of hemorrhage into the synovial membrane and subsequent release of hemoglobin pigment into the joint fluid. White-yellow discoloration indicates increased cellularity due to inflammation, sepsis, or neoplasia.
Normal synovial fluid is clear. Cloudy fluid can be due to elevated white blood cells, organisms, or fibrin. In rare cases cloudy fluid is due to presence of neoplastic cells or crystals.
Normal fluid is very viscous. A drop of fluid will form a string 2.5-5cm long when slowly dropped from the needle tip. Thin, runny fluid indicates fluid deficient in hyaluronic acid. This may be a result of decreased production due to synovial cell damage, dilution due to influx of plasma, or degradation by white blood cells or bacteria. The mucin clot test is a semiquantitative indicator of hyaluronic acid content, but is uncommonly performed because it adds little information that cannot be obtained from the "string test".
Protein
Total protein can be estimated by refractometer. Protein concentration is normally <2.5mg/dL (1.5-3.0). Increased protein concentration is seen with most inflammatory arthropathies. Concentration may be affected by excess EDTA/sample ratio in the tube. Decreased protein concentration can be seen after joint lavage.
Total nucleated cell counts
Total nucleated cell counts are used to diagnose inflammatory vs non-inflammatory arthropathies, and can help pinpoint the underlying cause of an inflammatory arthropathy. Serial testing can be used to assess response to treatment. For dogs, the upper limit of normal is usually 3000 cells/µL, although most normal joint fluid is significantly lower than this. In cats, up to 1000 cells/µL may be considered normal. Total cell counts can be obtained by automated counters or by hemacytometer. Fluid viscosity may interfere with counts, and can be reduced by adding 1-2 drops of hyaluronidase solution to the joint fluid.
Differential cell counts
Synovial fluid smears can be prepared similarly to blood smears. Again, viscosity may affect the 'smearability' of the fluid, and can alter the appearance of cells. Highly viscous fluid can be spread in a thinner layer by holding the smearing slide at a lower angle than normal. Normal smears will have a pink, coarsely granular background, with a folded appearance. Cells may appear smaller and darker than normal, and in some cases may not be readily identifiable. In highly cellular taps, the cells may line up in "windrows". The best location for evaluating cells is on the leading edge of the smear. In samples with decreased hyaluronic acid content, the background color and granularity will be decreased or non-existent.
Typically, neutrophils, lymphocytes, monocytes, and macrophages may be seen on synovial fluid smears. Normal fluid contains <10% neutrophils. Small lymphocytes, monocytes and macrophages make up the remaining 90%. In addition, there may be scattered synoviocytes in the smear. These usually appear as ovoid cells with basophilic cytoplasm and eccentric nuclei.
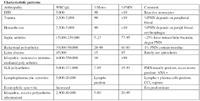
Characteristic patterns