
Pharmacology
Latest News
Latest Videos

Podcasts
More News

The long-lasting injection was first cleared in Australia and New Zealand in 2023.

Zoetis' Dectomax-CA1 is the first drug to receive conditional approval in the US for addressing this type of parasitic infestation.

The revision removes language about fatal vaccine-induced disease from the skin allergy drug's label.

The agency found that Gallant’s data provides reasonable expectation of effectiveness.
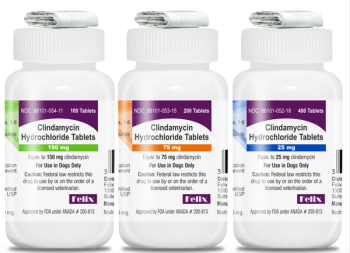
Felixvet's Clindamycin Hydrochloride Tablets gain FDA approval, offering a new oral antibiotic option for treating various canine infections.
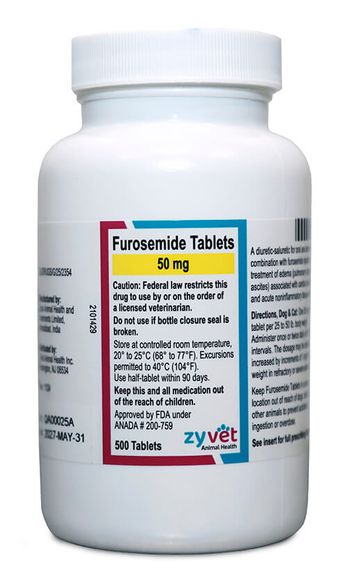
Generic furosemide is used to help manage heart failure.

Pegasus Laboratories celebrated the grand opening of a larger facility in Pensacola, Florida, with a ribbon-cutting ceremony.

The monoclonal antibody therapy (Portela; Zoetis) is designed to provide relief from pain associated with osteoarthritis.

Elanco Animal Health is pursuing international approvals for a parasite protection therapy and a dermatology drug.

The parasiticide also treats Dermanyssus gallinae, also known as red mites.

The therapy from Boehringer Ingelheim provides protection against porcine circovirus type 2.

Australian start-up Snoretox pitched the injectable therapy during the 2025 KC Animal Health Corridor Summit in Kansas City, Missouri.

Gamrozyne is the first generic version of Zactran, a pioneer product.

Michael W. Dryden, DVM, MS, PhD, DACVM (Parasitology), delivered a keynote address focused on canine heartworm disease on day 2 of the 2025 Fetch dvm360 Conference in Kansas City, Missouri.

The company voluntarily recalled its 2 lots of product due to potential health risks.

Regulatory approval from animal health authorities is still required before the vaccine can be made commercially available.

The JAK inhibitor will be available to veterinarians and dog owners in the United Kingdom by the end of Q3 2025.

Nostrum Laboratories cannot guarantee the safety of Sucralfate Tablets USP 1 gram manufactured after June 2023.

This latest approval builds on an earlier liquid formulation of generic methimazole for feline hyperthyroidism, which received FDA clearance earlier this month

Fluralaner for extended-release injectable suspension (Bravecto Quantum) is also indicated for treatment of lone star tick in dogs.

TruCan Ultra CIV H3N2/H3N8 will broadly protect canine patients from canine influenza.
The funding will support Gallant’s treatment for refractory feline chronic gingivostomatitis, which is in development.
Cefovecin Sodium for Injection is sponsored by Qilu Animal Health Products Co.

ELIAS Cancer Immunotherapy was combined with chemotherapy in a study with results presented at the 2025 ACVIM Forum.

Sirolimus delayed-release tablets (felycin-CA1; PRN Pharmacal) have conditional approval from the FDA.



















