A challenging case: Getting to the source of a dog's chronic spinal pain
A 14-month-old intact male Doberman pinscher-mix was presented to Texas A&M University's Texas Veterinary Medical Center for evaluation of chronic spinal pain and lameness.
A 14-MONTH-OLD intact male Doberman pinscher-mix was presented to Texas A&M University's Texas Veterinary Medical Center (TVMC) for evaluation of chronic spinal pain and lameness. The dog had been adopted as a stray eight months previously. The owner stated that the dog had exhibited intermittent, mild spinal pain that had progressively increased in intensity and frequency since adoption.
First presentation
Pain was elicited on palpation of the lumbosacral region. Findings from the remainder of the physical examination and a neurologic examination were unremarkable. No abnormalities were found on radiographic examination of the thoracic, lumbar, and sacral vertebrae, and we tentatively diagnosed lumbosacral stenosis. The owner declined additional diagnostic testing and elected conservative treatment, consisting of one month of strict cage rest and a tapering dose of oral prednisone: 2 mg/kg orally twice a day for three consecutive days, then once a day for three consecutive days, then every other day for three more doses. The dog's clinical signs improved within days of our initiating this treatment regimen, but recurred about three weeks into the course of treatment.

Vital Stats
Second presentation
The dog was presented to the TVMC four months after the initial visit for reevaluation. Since the previous visit, the owner reported that the dog had exhibited intense pain when its spine was manipulated. The spinal pain was now continuous, and the dog was moderately lame in the hindlimbs. Additionally, the animal's activity level had diminished, it had become inappetent, and the owner reported that the dog had lost weight.
Physical examination and diagnostic tests
On physical examination, the dog was mildly febrile (102.7 F [39.3 C]), thin (body condition score of 1.5 on a scale of 1 to 5), and exhibited weightbearing lameness in both hindlimbs. Intense pain was elicited on palpation of the caudal thoracic, lumbar, and sacral vertebral column. Conscious proprioception and spinal reflexes were normal in all four limbs. The scrotum and testicles were normal on palpation. On transrectal palpation, the prostate was unremarkable. Abdominal palpation and thoracic auscultation revealed no abnormalities. Peripheral lymph nodes were palpably normal. The ocular anterior chamber and fundic examination findings were normal.
Radiographic examination of the thoracic and lumbar spine showed osteolytic disease of the vertebral end plates with spondylosis associated with the intervertebral disk space at T5-T6 and L2-L3 (Figure 1). Lesser changes were present in the adjacent vertebral end plates at T13-L1, and equivocally at L3-L4 and L4-L5. Evidence of chronic irregularity of the articular facets was present at L3-L4 and L5-L6. Because of these radiographic findings, we diagnosed chronic diskospondylitis of the thoracic and lumbar spine with atypical degenerative joint disease of the lumbar spine.
1. A close-up view from a lateral thoracic and lumbar spinal radiograph showing the second and third lumbar vertebræ of an 18-month-old intact male Doberman pinscher-mix evaluated for chronic back pain and lameness. Osteolysis of the vertebral end plates (black arrows) with spondylosis associated with the intervertebral disk space (arrowheads) is present. There is evidence of chronic irregularity of the third lumbar articular facet (white arrow).
The results of a complete blood count and serum chemistry profile were normal except for thrombocytopenia (133 x 103 /µl; normal = 200 to 500 x 103 /µl). Urinalysis by cystocentesis revealed a urine specific gravity of 1.058, proteinuria (30 mg/dl; normal = 0 to trace), hematuria (1 to 3 RBC/hpf; normal = 0/hpf), pyuria (5 to 10 WBC/hpf; normal = 0 to 5/hpf), and a few squamous epithelial cells per low-power field. No bacteria were seen on sediment examination. Samples were submitted for aerobic and anaerobic bacterial blood and quantitative bacterial urine cultures and antimicrobial susceptibility testing. A blood sample was submitted for Brucella canis immunofluorescent antibody and tube agglutination tests.
Additional test results and treatment
Initial treatment, pending culture and serologic test results, consisted of cephalexin (30 mg/kg orally b.i.d.), etodolac (10 mg/kg orally once a day), and strict cage rest. The dog was discharged the same day. No improvement was noted after three days. Serologic titer results indicated exposure to B. canis (1:200 on the immunofluorescent antibody test and 1:100 on the tube agglutination test). Results of the aerobic blood culture revealed a Brucella species. A Brucella species (2,000 cfu/ml) was also identified on quantitative urine culture. Results of the anaerobic blood culture were negative. The Brucella species isolated was susceptible to all tested antimicrobials with the exception of trimethoprim-sulfadiazine. A sample of the cultured Brucella species was submitted for species identification (National Veterinary Services Laboratories, 1800 Dayton Ave., Ames, IA 50010), and the isolate was confirmed to be B. canis.
The owner elected to continue treatment after being advised of the zoonotic risk and guarded long-term prognosis. The owner was informed that dog-to-human transmission of B. canis is rare but that the owner should consult with a physician, avoid contact with the dog's body fluids (e.g. urine), and practice good hygiene after contact with the dog. We discontinued the cephalexin and initiated enrofloxacin (15 mg/kg orally once a day) and doxycycline hyclate (5 mg/kg orally b.i.d.) for a planned treatment course of at least 10 weeks. The etodolac therapy and cage rest were continued.
On our recommendation, the dog was castrated two days later. Bilateral testicular and epididymal tissue samples were submitted for aerobic culture. A Brucella species was isolated from all four samples. Both testes and epididymides were submitted for histologic evaluation. Scattered small, multifocal aggregates of plasma cells, lymphocytes, and occasional macrophages were present in the interstitium of the epididymides and between the seminiferous tubules (Figure 2). Occasional focal areas of inflammation between the seminiferous tubules contained intralesional spermatozoa (sperm granulomas). Multifocal areas of the testes contained degenerating and atrophied seminiferous tubules with spermatidic multinuclear giant cells. In the most profound areas of seminiferous tubular atrophy, increased interstitium and scattered interstitial macrophages and fibroblasts surrounded remnants of the tubules. The histologic findings of testicular atrophy with orchitis and epididymitis were present bilaterally, but the atrophy was more extensive in the left testicle.
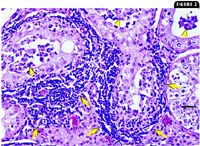
2. A photomicrograph of a section of testicle. Lymphocytes and plasma cells are present in the interstitium (arrows) between seminiferous tubules and are multifocally infiltrating the tubules (arrowheads) (hematoxylin-eosin; bar = 100 µm).
Follow-up
About a week after the antimicrobial therapy was changed and the castration performed, the owner reported that the dog's lameness had resolved and the spinal pain had greatly diminished. On recheck examination at the TVMC four weeks after the diagnosis, lameness was not evident, and palpation along the vertebral column revealed no pain. The dog had gained 4 lb (1.8 kg) and was no longer febrile. The owner reported that the dog's activity level and appetite had markedly increased in the previous weeks. On repeat vertebral radiographs, there was increased radiopacity of the previously noted lytic lesions of the thoracic and lumbar vertebrae. The etodolac therapy was discontinued at this time.
At a second recheck examination 10 weeks after the diagnosis, the dog was asymptomatic and had gained another 4 lb. We again obtained radiographs of the affected regions of the vertebral column. The lumbar vertebral lesions showed further improvement; the lytic lesions were becoming less apparent. The thoracic vertebral lesions were unchanged compared with the previous radiographs. We advised the owner to continue treatment, but the owner elected to discontinue antimicrobial therapy. To monitor for recurrence, we recommended recheck examinations and vertebral radiography every three months for one year or immediately if any of the original clinical signs were observed.
Third presentation
Four months after the antimicrobial therapy was discontinued, the dog was presented to the TVMC for evaluation of spinal pain and lethargy. The owner reported that the dog had been active and nonpainful for the previous 16 weeks and that the onset of the current signs was acute. On physical examination, severe pain was noted on palpation of the caudal thoracic and lumbosacral vertebral columns. The dog's body weight was unchanged from the previous examination. Its rectal temperature was normal, and no abnormalities were identified on neurologic examination.
Diagnostic tests and treatment
A thoracic and lumbosacral vertebral radiographic examination was repeated, but no changes were noted compared with the radiographs obtained on the previous visit. Urine was collected by cystocentesis for analysis and quantitative bacterial culture and antimicrobial susceptibility testing. Blood was collected for B. canis serology and aerobic bacterial culture. The results of urinalysis and a sediment examination were unremarkable. A Brucella species (800 cfu/ml) was isolated on urine culture. A Brucella species was also identified on aerobic blood culture. The antimicrobial susceptibility of these isolates was identical to the B. canis cultured on initial presentation. Serologic titer results indicated exposure to B. canis (1:200 on both the immunofluorescent antibody and tube agglutination tests).
After we discussed the long-term prognosis and zoonotic concerns, the owner elected to restart treatment. Therapy with enrofloxacin (15 mg/kg orally once a day) and doxycycline (5 mg/kg orally b.i.d.) was reinitiated for an empirically chosen treatment course of at least 12 months. Etodolac (10 mg/kg orally once a day) and strict cage rest were recommended for one month. As before, the dog responded rapidly and dramatically to treatment. The owner reported that the dog was asymptomatic after one week of antimicrobial therapy.
Follow-up
After this recurrence of the dog's clinical signs, three recheck examinations have been performed about every three months. Over this period, the dog has remained asymptomatic. During each recheck, palpation of the spine has revealed no pain, and the dog has remained afebrile. It has gained about 2 lb (0.9 kg) at each recheck.
On the first recheck during this period, radiographs of the thoracic and lumbar spine showed no changes in the lumbar diskospondylitis lesions, but increased bone lysis was present at T5-T6. Treatment at this time was unaltered except for increasing the enrofloxacin dose to adjust for weight gain. On the second recheck examination, immunofluorescent antibody and tube agglutination tests were repeated, and the results of each were 1:200. The dog's body condition score was 4/5, and the treatment was unaltered. On the third recheck, immunofluorescent antibody and tube agglutination test results had decreased to 1:100 (interpreted as a weak seropositive result), and antimicrobial treatment was continued.
The dog has not been rechecked in the subsequent four months but continues to receive antimicrobial therapy. The owner reported that the dog has remained asymptomatic and is doing well.
Discussion
Diskospondylitis is an infection of the intervertebral disk and adjacent vertebral bodies. It most commonly affects large- and giant-breed dogs.1 Males are affected twice as frequently as females.2 The most common clinical sign is spinal pain, which is present in more than 80% of cases.3 Other clinical signs are more variable and include anorexia, weight loss, lethargy, fever, and neurologic deficits.4
Hematogenous spread of organisms from local or systemic infection is thought to be the most common cause of diskospondylitis.1 Conditions that may contribute to diskospondylitis include urogenital tract infections, bacterial endocarditis, periodontal disease, and skin infections.1,5,6 Other potential sources of infectious organisms include penetrating skin wounds, migrating foreign bodies, vertebral surgery, and extension of paravertebral infections.1,7-9 A variety of bacterial organisms have been isolated from diskospondylitis lesions; Staphylococcus and Streptococcus species, Escherichia coli, and B. canis have been reported most frequently, and Pasteurella haemolytica, Pseudomonas aeruginosa, and Enterococcus, Nocardia, Actinomyces, Micrococcus, Proteus, and Corynebacterium species have been reported sporadically.7,8,10-12 Rarely, fungal organisms have been identified, including Aspergillus, Fusarium, Mucor, Penicillium, Paecilomyces, Chrysosporium, and Pseudallescheria species.10,13 Brucella canis has been implicated as the causative organism in 12.5% and 10.4% of canine diskospondylitis cases in two studies.4,14
Brucella canis is a small, gram-negative, aerobic coccobacillus.15 It can cause systemic infections in dogs, with a variety of clinical manifestations. These clinical abnormalities are the result of organism localization within tissues and immune-complex-associated pathology.16 Abortions and infertility resulting from placental infections, orchitis, and epididymitis are the most common clinical abnormalities associated with infection.17 Enlarged lymph nodes, uveitis, diskospondylitis, osteomyelitis, meningitis, glomerulonephritis, and dermatitis have also been reported.14,17-20 It is interesting that no clinical signs attributable to the urinary, lymphatic, or reproductive tract were present in this case, as would be expected in an animal infected with B. canis. Histologic examination of the testes and epididymides confirmed extensive pathology, but no abnormalities were found on clinical examination.
Brucella canis infection can be transmitted between dogs by several routes. Infections may be acquired by ingestion, by inhalation of aerosolized organisms, by venereal contact, in utero, and by fomites (e.g. from instruments used during artificial insemination or blood transfusion).15 Organisms can be found in vaginal discharge, seminal fluid, aborted materials, urine, milk, saliva, and nasal secretions from infected individuals.15,21,22 Natural infection is thought to most commonly occur by oronasal contact with aborted materials.
A diagnosis of B. canis infection carries many important clinical implications. Brucella canis is a potential zoonotic agent. More than 35 human infections with B. canis have been reported in the literature, but people appear relatively resistant to infection, and the disease is typically responsive to antimicrobial therapy.23,24 The mechanism of B. canis transmission from dogs to people has not been fully elucidated, but it is thought to occur by contact with infected tissues or body fluids.24 Typical clinical signs in people include fatigue, fever, chills, weight loss, and enlarged lymph nodes.24-26 Rarely, serious complications, such as meningitis, endocarditis, hepatitis, arthritis, osteomyelitis, and visceral abscesses, may develop.23,27
An additional complicating factor in the management of these animals is that treatment of B. canis infections is difficult and requires long-term antimicrobial therapy. Even when appropriate antimicrobial regimens and treatment durations are used, relapses of clinical signs are common.14 No treatment protocols have been shown to completely and permanently clear dogs of infection. Using a combination of dihydrostreptomycin and doxycycline, long considered the treatment of choice for canine brucellosis, is no longer possible because of the limited availability of dihydrostreptomycin.28 Dihydrostreptomycin and other aminoglycoside substitutes also require parenteral administration, which can make treatment with these agents impractical for some owners. The combination of fluoroquinolones and tetracyclines has shown promise in vitro, but clinical data supporting its efficacy are lacking.29 Appropriate antimicrobial treatment durations have not yet been adequately documented in dogs with brucellosis. Resolution of clinical signs, cessation of bacteremia, and repeatable seronegative status may be used to guide treatment, but elimination of infection is difficult to confirm antemortem. These ramifications make accurate diagnosis of the causative agent of diskospondylitis critical. Surgical intervention may be warranted in dogs with diskospondylitis that have severe neurologic signs and fail to respond to conservative treatment or when vertebral instability or spinal cord compression is present. The lesion's site, extent, and surgical accessibility must also be considered.
Serologic testing is commonly used to screen for B. canis infection, but questionable test validity and interpretation make obtaining an accurate diagnosis difficult.30 When serologic tests are used, it is important to interpret the results with knowledge of each test's sensitivity and specificity. Numerous serologic tests are available; the most commonly used tests are the 2-mercaptoethanol rapid slide agglutination test (2-ME-RSAT), 2-ME tube agglutination test (2-ME-TAT), and agarose gel immunodiffusion (AGID) test. Each of these tests has relative strengths and weaknesses. The 2-ME-RSAT and 2-ME-TAT are considered screening tests and are highly sensitive for infection. However, test results are often negative during the first five to eight weeks of infection, and both tests are troubled by cross-reactivity with antibodies to other bacterial species, often leading to false positive results.30 The 2-ME-TAT provides semiquantitative results, which can be useful in monitoring a patient's response to therapy. Two types of AGID tests are available: the AGID1 uses cell wall antigens and the AGID2 uses cytoplasmic antigens.30 The AGID1 test is highly sensitive and produces positive results in a similar time frame to that of the 2-ME-RSAT and 2-ME-TAT, but nonspecific reactions can result in false positive results.31 The AGID2 is the least sensitive of the commonly used serologic tests, and it does not routinely produce positive results until eight to 12 weeks after infection.31 However, it is highly specific for Brucella species, making it a good confirmatory test.31 An additional advantage of the AGID2 test is that the results remain positive for at least 12 months after the cessation of bacteremia (which may occur as early as six months after infection), while the agglutination and AGID1 tests may become diagnostically insignificant or give equivocal results during this period.32
This case emphasizes the importance of body fluid and tissue culturing. Although we are unaware of any other reports of B. canis being cultured simultaneously from the blood, urine, and reproductive tissues of a dog with diskospondylitis, routine cultures are of clinical value. Brucella canis can be isolated from several sites, including blood, urine, semen, vaginal discharge, gonadal tissue, intervertebral disk material, and cerebrospinal fluid.10,14,21,33,34 In several studies, blood culture results were positive in 45% to 75% of dogs with diskospondylitis, and urine culture results were positive in 25% to 60% of dogs.35-37 A report of B. canis diskospondylitis found blood culture results were positive in 30% of cases, and urine culture results were positive in 33% of cases.14
Characteristic radiographic signs of diskospondylitis include disk space collapse, bone lysis in the end plate region, end plate sclerosis, and variable bone proliferation.1,4,10 The severity and progression of radiographic lesions are highly variable and correlate poorly with clinical signs.1 It is important to radio-graph the vertebral column cranial and caudal to the lesions to accurately define the extent of vertebral involvement.
Conclusion
Brucella canis is a relatively common cause of canine diskospondylitis, and a diagnosis of brucellosis is associated with many clinical challenges. Although B. canis infection associated with diskospondylitis has been well-documented in the literature, actual reports of cases and their associated management have been minimal and brief, and detailed case management has not been reported in almost 20 years.38,39 Much has been reported since that time regarding the pathophysiology of B. canis infection, its zoonotic potential, and the difficulties associated with treating individual family-owned pet dogs.
Determining the causative organism in cases of canine diskospondylitis is essential because of the zoonotic potential and treatment difficulties associated with brucellosis. It is important to use a combination of diagnostic modalities, including cultures, serologic tests, and clinical findings. Treatment for B. canis infections should only be undertaken with the understanding that a cure should not be expected, long-term antimicrobials will be required, and recrudescence of infection is common. The dog in this case continued to have active diskospondylitis lesions even after several months of continuous antimicrobial therapy.
On histologic examination, this dog had dramatic testicular and epididymal damage despite a complete lack of clinical abnormalities of the reproductive tract. This emphasizes the need for early castration of male dogs if treatment is a consideration. Neutering infected dogs is recommended to reduce the risk of transmission to other dogs and people, remove potential reservoirs of infection, and resolve clinical abnormalities (e.g. orchitis, epididymitis).14 An additional point of interest in this case is the late development of radiographic signs of diskospondylitis. Presumably, the spinal pain in this dog that the owner had observed for the eight months before presentation was due to B. canis infection, yet the development of radiographic signs of bone lysis was delayed long past that usually associated with other bacterial causes of diskospondylitis. Typically, radiographic changes become evident within three weeks after the onset of clinical signs.1
Always consider brucellosis in dogs with clinical signs compatible with diskospondylitis. When present, the variety of systemic clinical manifestations associated with B. canis infection can help to increase the index of suspicion for canine brucellosis. As this case illustrates, however, the presence and clinical detectability of these systemic abnormalities are highly variable and should not be used to rule out B. canis infection.
Eric C. Ledbetter, DVM
Department of Clinical Sciences
College of Veterinary Medicine
Cornell University
Ithaca, NY 14853-6401
Sharon C. Kerwin, DVM, MS, DACVS
Department of Small Animal Medicine and Surgery
College of Veterinary Medicine
Texas A&M University
College Station, TX 77843-4457
Joanne K. Mansell, DVM, MS, DACVP
Department of Pathobiology
College of Veterinary Medicine
Texas A&M University
College Station, TX 77843-4457
George A. Henry, DVM, DACVR*
Department of Large Animal Medicine and Surgery
College of Veterinary Medicine
Texas A&M University
College Station, TX 77843-4457
*Current address: Department of Small Animal Clinical Sciences
College of Veterinary Medicine
The University of Tennessee
Knoxville, TN 37996
REFERENCES
1. Kornegay, J.N.; Barber, D.L.: Diskospondylitis in dogs. JAVMA 177 (4):337-341; 1980.
2. Kornegay, J.N.: Canine diskospondylitis. Compend. Cont. Ed. 1 (12):930-933; 1979.
3. Thomas, W.B.: Diskospondylitis and other vertebral infections. Vet. Clin. North Am. (Small Anim. Pract.) 30 (1):169-182; 2000.
4. Gilmore, D.R.: Lumbosacral diskospondylitis in 21 dogs. JAAHA 23 (1):57-61; 1987.
5. Calvert, C.A. et al.: Cardiovascular infections in dogs: Epizootiology, clinical manifestations, and prognosis. JAVMA 187 (6):612-616; 1985.
6. Turnwald, G.H. et al.: Diskospondylitis in a kennel of dogs: Clinicopathologic findings. JAVMA 188 (2):178-183; 1986.
7. Moore, M.P.: Discospondylitis. Vet. Clin. North Am. (Small Anim. Pract.) 22 (4):1027-1034; 1992.
8. Johnston, D.E.; Summers, B.A.: Osteomyelitis of the lumbar vertebrae in dogs caused by grass-seed foreign bodies. Aust. Vet. J. 47 (7):289-294; 1971.
9. Remedios, A.M. et al.: Epidural abscess and discospondylitis in a dog after administration of a lumbosacral epidural analgesic. Can. Vet. J. 37 (2):106-107; 1996.
10. Hurov, L. et al.: Diskospondylitis in the dog: 27 cases. JAVMA 173 (3):275-281; 1978.
11. Adamo, P.F.; Cherubini, G.B.: Discospondylitis associated with three unreported bacteria in the dog. J. Small Anim. Pract. 42 (7):352-355; 2001.
12. Mitten, R.W.: Vertebral osteomyelitis in the dog due to Nocardia-like organisms. J. Small Anim. Pract. 15 (9):563-570; 1974.
13. Watt, P.R. et al.: Disseminated opportunistic fungal disease in dogs: 10 cases (1982-1990). JAVMA 207 (1):67-70; 1995.
14. Kerwin, S.C. et al.: Diskospondylitis associated with Brucella canis infection in dogs: 14 cases (1980-1991). JAVMA 201 (8):1253-1258; 1992.
15. Carmichael, L.E.; Greene C.E.: Canine brucellosis. Infectious Diseases of the Dog and Cat, 2nd Ed. (C.E. Greene, ed.). W.B. Saunders, Philadelphia, Pa., 1998; pp 248-257.
16. Carmichael, L.E.; Kenney, R.M.: Canine brucellosis: The clinical disease, pathogenesis, and immune response. JAVMA 156 (12):1726-1734; 1970.
17. Hubbert, N.L. et al.: Canine brucellosis: Comparison of clinical manifestations with serologic test results. JAVMA 177 (2):168-171; 1980.
18. Smeak, D.D. et al.: Brucella canis osteomyelitis in two dogs with total hip replacements. JAVMA 191 (8):986-990; 1987.
19. Dawkins, B.G. et al.: Pyogranulomatous dermatitis associated with Brucella canis infection in a dog. JAVMA 181 (11):1432-1433; 1982.
20. Riecke, J.A; Rhoades, H.E.: Brucella canis isolated from the eye of a dog. JAVMA 166 (6):583-584; 1975.
21. George, L.W. et al.: Semen examination in dogs with canine brucellosis. AJVR 40 (11):1589-1595; 1979.
22. Carmichael, L.E.; Joubert, J.C.: Transmission of Brucella canis by contact exposure. Cornell Vet. 78 (1):63-73; 1988.
23. Young, E.J.: Human brucellosis. Rev. Infect. Dis. 5 (5):821-842; 1983.
24. Munford, R.S. et al.: Human disease caused by Brucella canis. A clinical and epidemiologic study of two cases. JAMA 231 (12):1267-1269; 1975.
25. Polt, S.S. et al.: Human brucellosis caused by Brucella canis: Clinical features and immune response. Ann. Intern. Med. 97 (5):717-719; 1982.
26. Rumley, R.L.; Chapman, S.W.: Brucella canis: An infectious cause of prolonged fever of undetermined origin. South. Med. J. 79 (5):626-628; 1986.
27. Piampiano, P. et al.: Brucellosis: Unusual presentations in two adolescent boys. Pediatr. Radiol. 30 (5):355-357; 2000.
28. Nicoletti, P.: Further studies on the use of antibiotics in canine brucellosis. Compend. Cont. Ed. 13 (6):944-947; 1991.
29. Mateu-de-Antonio, E.M.; Martin, M.: In vitro efficacy of several antimicrobial combinations against Brucella canis and Brucella melitensis strains isolated from dogs. Vet. Microbiol. 45 (1):1-10; 1995.
30. Carmichael, L.E.; Shin, S.J.: Canine brucellosis: A diagnostician's dilemma. Semin. Vet. Med. Surg. (Small Anim.) 11 (3):161-165; 1996.
31. Johnson, C.A.; Walker, R.D.: Clinical signs and diagnosis of Brucella canis infection. Compend. Cont. Ed.14 (6):763-772; 1992.
32. Carmichael, L.E. et al.: Problems in the serodiagnosis of canine brucellosis: Dog responses to cell wall and internal antigens of Brucella canis. Dev. Biol. Stand. 56:371-383; 1984.
33. Serikawa, T. et al.: Significance of urine-culture for detecting infection with Brucella canis in dogs. Jpn. J. Vet. Sci. 40 (3):353-355; 1978.
34. Schoeb, T.R.; Morton, R.: Scrotal and testicular changes in canine brucellosis: A case report. JAVMA 172 (5):598-600; 1978.
35. Kornegay, J.N.: Diskospondylitis. Textbook of Small Animal Surgery, 2nd Ed. (D. Slatter, ed.). W.B. Saunders, Philadelphia, Pa., 1993; pp 1087-1094.
36. Kornegay, J.N.: Diskospondylitis. Current Veterinary Therapy IX Small Animal Practice (R.W. Kirk, ed.). W.B. Saunders, Philadelphia, Pa., 1986; pp 810-814.
37. Fischer, A. et al.: Fluoroscopically guided percutaneous disk aspiration in 10 dogs with diskospondylitis. J. Vet. Intern. Med. 11 (5):284-287; 1997.
38. Henderson, R.A. et al.: Discospondylitis in three dogs infected with Brucella canis. JAVMA 165 (5):451-455; 1974.
39. Anderson, G.I.; Binnington, A.G.: Discospondylitis and orchitis associated with high brucella titre in a dog. Can. Vet. J. 24:249-252; 1983.