Exercise intolerance in retrievers
Retrievers typically have an active lifestyle, and many are engaged in hunting, field competition, or other strenuous activities such as agility or search and rescue work.
Retrievers are among the most popular dog breeds in the world. The Labrador retriever has been the most commonly registered breed in both the American Kennel Club and the Canadian Kennel Club for more than a decade, and the golden retriever consistently ranks in the top five to 10 breeds.1,2 Retrievers typically have an active lifestyle, and many are engaged in hunting, field competition, or other strenuous activities such as agility or search and rescue work. Retrievers presented to veterinarians for perceived exercise intolerance may have decreased strength, speed, or stamina compared with other dogs or compared with their own usual performance standard. Signs can sometimes be subtle, with suboptimal performance detected by the owner or handler, but not detectable by inexperienced observers. Alternatively, the signs may include profound exercise-induced weakness, episodes of collapse, or even death following exercise.
This article focuses on the conditions associated with exercise intolerance in retrievers. The conditions and the principles of evaluating these patients that we outline apply to other breeds of dogs as well, but we emphasize those disorders most likely to be found in the retriever breeds.
HISTORY TAKING
A complete history investigating abnormalities in every body system is important (Table 1). Whenever possible, a veterinarian should observe the dog while it is manifesting what the owner perceives as exercise intolerance. When this is not feasible, details regarding the type, duration, and intensity of exercise that result in exercise intolerance and a clinical description of the exercise intolerance itself should be collected from the owner or handler. In some cases, a videotape of the dog exercising can help to characterize the perceived exercise intolerance.

Table 1. Exercise Intolerance Supplemental History
PHYSICAL EXAMINATION
Many dogs with exercise intolerance have abnormal physical examination findings at rest. Careful physical examination may detect abnormalities that lead to a diagnosis. Complete respiratory, cardiovascular, musculoskeletal, and nervous system examinations should be performed, as well as thorough abdominal palpation. When the patient history, physical examination, and routine laboratory evaluation do not provide a diagnosis, it may be necessary to exercise the dog in order to examine it while it is exercise intolerant.
CONDITIONS ASSOCIATED WITH EXERCISE INTOLERANCE
Lack of conditioning or obesity
Some dogs presented for exercise intolerance are simply out of condition. Working dogs asked to perform without much preparatory conditioning may tire quickly and be reluctant to exercise. Dogs may not be acclimatized to extremes of heat or humidity, making it difficult to exercise without overheating.
Obesity is common in dogs, especially retrievers, and can be associated with a variety of medical disorders and orthopedic problems that can lead to exercise intolerance.3 Obese retrievers have also been shown to have small airway collapse during expiration, limiting their ability to exercise.4 Weight loss in obese dogs with hip osteoarthritis substantially improves their gait and their ability to exercise.3
Bone and joint disorders
Discomfort from abnormalities of the bones or joints causes reluctance to exercise. Lameness will not always be apparent, particularly if more than one limb is affected. Generalized stiffness and reluctance to exercise at full capacity may be the only findings in dogs with painful disorders affecting multiple bones or joints. Young dogs suffering from panosteitis, hypertrophic osteodystrophy, or osteochondritis dissecans (OCD) all show a reluctance to exercise. Older dogs with ligamentous injuries or degenerative joint disease will be similarly affected.5
Inflammatory disease affecting multiple joints, termed polyarthritis, causes joint pain and reluctance to exercise in dogs.6 Although some cases can be attributed to tick-borne infectious diseases (e.g. ehrlichiosis, Lyme disease, granulocytic anaplasmosis, bartonellosis, Rocky Mountain spotted fever), noninfectious idiopathic immune-mediated nonerosive polyarthritis (IMPA) is the most common cause of polyarthritis in dogs.6-8 Labrador retrievers and Nova Scotia duck tolling retrievers may be predisposed.6,9 In dogs with IMPA, immune complex deposition within the synovium results in a sterile synovitis. Clinical signs include reluctance to walk or exercise, fever, and lethargy. Lameness, joint swelling, and joint pain can be inconsistent, so diagnosis requires arthrocentesis with cytology and bacterial culture.6-9
Degenerative lumbosacral stenosis
The cauda equina is the collection of spinal nerve roots descending from the end of the spinal cord within the vertebral canal to their point of exit from the canal. Dogs with compression of these nerves from degenerative lumbosacral stenosis exhibit exercise intolerance and a reluctance to run or jump.10 Degenerative lumbosacral stenosis is a common disorder in aging retrievers, with cauda equina compression caused by acquired Hansen's type II disk prolapse at L7-S1, bone remodeling, and soft tissue proliferation.10 Progressive narrowing of the lumbosacral vertebral canal causes compression of the L7, sacral, and caudal nerve roots, resulting in a characteristic constellation of clinical signs.10-12 Affected dogs are slow to rise from a prone position and reluctant to run, sit up, jump, or climb stairs. Rear limb lameness and weakness commonly worsen with exercise, as the blood vessels accompanying the spinal nerve roots within the already crowded intervertebral foramen dilate, further compressing the nerve roots—a phenomenon termed neurogenic intermittent claudication.10,12,13 Some dogs will become obviously lame with exercise, while others will simply quit exercising because of the discomfort.
The most consistent physical examination abnormality in dogs with cauda equina syndrome is pain elicited by deep palpation of the dorsal sacrum, dorsiflexion of the tail, or hyperextension of the lumbosacral region.10,13 In severely affected dogs, there may also be rear limb weakness, atrophy of the muscles of the caudal thigh and distal limb, and incomplete hock flexion during the withdrawal reflex.10,12,13 Clinical findings are often the primary basis for reaching a diagnosis in affected dogs. Spinal radiographs are useful to rule out other lesions in the region such as diskospondylitis, lytic vertebral neoplasia, and vertebral fracture or luxation, and to identify predisposing factors for degenerative stenosis such as sacral osteochondrosis or vertebral malformations. When available, magnetic resonance imaging (MRI) provides the most sensitive, accurate, and noninvasive means of evaluating the lumbosacral region, allowing visualization of all components potentially involved in cauda equina compression.10-13 Restricting exercise and administering analgesics or anti-inflammatory drugs may temporarily improve the condition in some dogs with clinical signs limited to pain and lameness. More definitive treatment involves lumbosacral dorsal laminectomy, excision of compressing tissues, and L7-S1 foraminotomy when necessary.10-13
Cardiovascular disorders
Dogs may be presented for acquired exercise intolerance caused by cardiovascular dysfunction. Dogs with heart failure from congenital anomalies, acquired valvular heart disease, or cardiomyopathy will be unable to exercise because of poor perfusion and tissue hypoxia. These dogs will typically exhibit physical evidence of cardiac failure at rest, including tachycardia, cough, weak femoral pulses, crackles on lung auscultation from pulmonary congestion or edema, and perhaps cyanosis and a murmur.
The most common congenital heart defects in retrievers include subvalvular aortic stenosis (SAS) in golden retrievers and tricuspid valve dysplasia (TVD) in Labrador retrievers.14,15 Many golden retrievers with mild SAS can live a normal lifespan with normal exercise tolerance, but more severely affected dogs are at risk for sudden death, arrhythmias, and congestive heart failure.14 Most Labrador retrievers with TVD have a shorter than normal lifespan (mean of 2 years), with affected dogs developing exercise intolerance, right-sided heart failure, and arrhythmias.14,15
Cardiac rhythm disturbances can cause episodic weakness, which may or may not be associated with periods of exercise. Tachyarrhythmias and bradyarrhythmias can cause serious reductions in cardiac output resulting in weakness, syncope, or sudden death. Auscultation, thoracic radiographs, electrocardiography, and echocardiography will be normal at rest in many dogs with arrhythmia-associated exercise intolerance or syncope.16 Cardiac event recording is a useful tool in these dogs to determine whether a cardiac arrhythmia is the cause of their clinical signs.16 Labrador retrievers are a breed at risk for high-grade second-degree and third-degree atrioventricular (AV) block, which can lead to exercise intolerance, episodic weakness, syncope, and sudden death.17 Pacemaker implantation is recommended in affected dogs to stabilize cardiac output. Neurocardiogenic (vasovagal) bradycardia with syncope occasionally occurs in otherwise normal golden retrievers during periods of exercise and excitement.18
Dogs with pericardial effusion causing cardiac tamponade are often presented to a veterinarian for evaluation of exercise intolerance, weakness, or collapse.19 Acquired pericardial effusion sufficient to cause tamponade and exercise intolerance is usually due to neoplasia, but idiopathic effusions can also occur.20 Hemangiosarcoma arising from the right atrial appendage or the wall of the right atrium is the most common neoplastic cause in retrievers, particularly in golden retrievers, which are predisposed to this neoplasia.19
When the pericardial effusion develops quickly, even a relatively small volume of fluid can prevent diastolic filling and dramatically decrease cardiac output, but pericardial effusion that accumulates slowly must be a high volume before tamponade occurs.20 Muffled heart sounds, tachycardia, jugular venous distention, and poor pulse quality are common physical findings.19 Pulsus paradoxus may be detected, with a weak femoral arterial pulse during inspiration and a stronger pulse during expiration.19 With chronic effusions, additional consequences of right-sided congestive heart failure may develop, including ascites and pleural effusion.
Cardiac tamponade is suspected based on clinical findings, but echocardiography is required to confirm the effusions and demonstrate diastolic collapse of the right atrium or ventricle consistent with tamponade. Transthoracic two-dimensional echocardiography has an 80% sensitivity for the detection of cardiac masses causing pericardial effusion and tamponade.19
Average survival in dogs with pericardial effusion secondary to right atrial hemangiosarcoma is one to three months. Idiopathic pericardial effusion may spontaneously resolve after therapeutic pericardiocentesis or may recur, requiring pericardiectomy, but the prognosis is good, with 72% survival after 18 months.20
Respiratory disorders
Abnormalities of the larynx, pharynx, trachea, airways, pulmonary parenchyma, or pleural space can impair a dog's ability to ventilate normally. As the affected dog exercises, it may be unable to keep up with the oxygen demands of the tissues, resulting in signs of weakness, exercise intolerance, or collapse. Auscultation and observation of the respiratory pattern at rest and during and after exercise can be useful in localizing a problem within the respiratory tract.21 Obtain thoracic radiographs in all dogs with exercise intolerance. Also, further diagnostic tests to evaluate the respiratory system should be performed as necessary to reach a diagnosis (Table 2).

Table 2. Respiratory Disorders That Cause Exercise Intolerance in Retrievers
Laryngeal paralysis. Laryngeal paralysis is a common disorder in geriatric large-breed dogs, particularly Labrador retrievers.22-24 Most cases are idiopathic—with no detectable underlying cause. Affected dogs may be mildly symptomatic for months, with inspiratory noise (stridor) and dyspnea that are only apparent during exercise or when the dog is overheated. Some owners also notice a chronic mild cough and a voice change.
Acute treatment of dogs presenting with severe stridor includes oxygenation, cooling, corticosteroids (dexamethasone 0.1 mg/kg intravenously), and tranquilization (acepromazine 0.005 to 0.02 mg/kg intravenously and butorphanol 0.2 mg/kg intravenously).21 Once an affected dog is stabilized medically, it should be evaluated systemically for an underlying cause or concurrent disease.
Routine screening blood tests, thoracic radiography, and a complete nervous system examination should always be performed. Additional tests may be warranted to rule out hypothyroidism, myasthenia gravis, and a generalized polyneuropathy.22,25 Diagnosis of laryngeal paralysis requires laryngoscopy using an anesthetic protocol that will not abolish laryngeal movement (Table 3). The respiratory stimulant doxapram can be administered to improve respiratory efforts during evaluation.22 Treatment of idiopathic laryngeal paralysis involves unilateral arytenoid lateralization (tie-back).22,23,26

Table 3. Anesthetic Protocol for Evaluating Laryngeal Function
The prognosis after surgery is good for large-breed dogs with idiopathic laryngeal paralysis, with 90% of owners pleased with their dogs' improvement one year postoperatively and a median survival of one to five years.23-26 Aspiration pneumonia is a potential complication. Some Labrador retrievers with laryngeal paralysis will, over time, develop clinical evidence of a progressive generalized peripheral neuropathy that will lead to exercise intolerance and weakness in spite of successful resolution of their respiratory obstruction.25
Anemia
Dogs will not usually manifest weakness or exercise intolerance due to anemia unless their hematocrit has fallen acutely to less than 20%. Acute severe anemia from trauma, ruptured splenic hemangiosarcoma, bleeding intestinal lymphoma, gastric ulceration, anticoagulant rodenticide intoxication, thrombocytopenia, or acute hemolysis typically results in sudden collapse or profound weakness rather than repeated episodes of inability to exercise. Chronic anemia is better tolerated but, when severe, can result in classic signs of exercise intolerance.27 Chronic anemia is most often seen with low-grade gastrointestinal or urinary bleeding, neoplasia, chronic hemolysis, or bone marrow disease. Perform a complete blood count in all dogs with exercise intolerance.
Hypoglycemia
Hypoglycemia is an important cause of weakness and exercise intolerance in dogs. In adult dogs, hypoglycemia is most likely caused by insulin-secreting neoplasms, other tumors, liver failure, hypoadrenocorticism, sepsis, or xylitol intoxication (Table 4).28-30 An inherited glycogen storage disease caused by a mutation of the glycogen debranching enzyme gene causes intermittent hypoglycemia in affected young and adult curly coated retrievers.31 Low blood sugar as a cause of exercise intolerance is best documented during an episode of weakness or when repeated hourly blood glucose samples are evaluated during fasting.28,29

Table 4. Causes of Hypoglycemia in Adult Retrievers
Functional islet cell tumors (insulinomas) are the most common causes of hypoglycemia in middle-aged and older dogs of all breeds.29 Signs of weakness, trembling, abnormal behavior, and disorientation are initially episodic but become more frequent with time and are often worse with exercise. Blood glucose concentrations may be normal when the dog is asymptomatic.28,29 Profound hypoglycemia is most likely to be demonstrated after a 24- or 48-hour fast or when measured 15 to 30 minutes after feeding, since a rise in blood glucose stimulates tumor insulin hypersecretion. Documenting normal or elevated serum insulin concentrations in the face of hypoglycemia supports a diagnosis of insulinoma.29 Thoracic radiographs and abdominal ultrasonography are recommended to look for metastatic disease. Treatment usually consists of partial pancreatectomy to remove the tumor followed by medical management using frequent small high-protein meals, prednisone, diazoxide, and other medical therapies designed to increase blood glucose or decrease insulin production or release.29
Symptomatic hypoglycemia of hunting dogs is a well-recognized but poorly characterized disorder seen in young adult, highly excited, thin, upland hunting dogs, especially pointers and spaniels.28 Episodes are characterized by apparent disorientation, weakness, and occasionally generalized seizures after one to two hours of vigorous exercise. Affected dogs are usually normal by the time they are evaluated by a veterinarian, so hypoglycemia can only be documented by collecting a blood sample in the field at the time of collapse. If hypoglycemia is confirmed, affected dogs should be vigorously evaluated for hypoadrenocorticism, liver dysfunction, and neoplasia.28,29,32 Excitement and intense exercise induce hyperglycemia in normal dogs, so there is speculation that dogs experiencing exercise-induced hypoglycemia may have a glycogen storage disorder.28,33,34 Feeding affected dogs in the morning before a hunt, frequent feeding of protein-rich foods during the hunt, or the administration of a small amount of prednisone (2.5 to 5 mg/dog) just before the hunt may prevent symptomatic hypoglycemia.28
Hypoadrenocorticism
Primary hypoadrenocorticism (Addison's disease) is a clinical syndrome resulting from a deficiency of cortisol and aldosterone.35 Hypoadrenocorticism is most common in young and middle-aged female dogs, with an increased prevalence and autosomal recessive inheritance in standard poodles, Portuguese water dogs, and Nova Scotia duck tolling retrievers.36 Golden retrievers and Labrador retrievers may also be predisposed to the disease.
Clinical signs of hypoadrenocorticism are typically related to fluid and electrolyte imbalance, circulatory insufficiency, and abnormal carbohydrate metabolism.35 Inappetence, lethargy, vomiting, and diarrhea are common. Muscular weakness or seizures that occur with exercise may be the only presenting complaint, presumably caused by hypoglycemia.32,35
Hyponatremia, hyperkalemia, and increased blood urea nitrogen concentration are common findings in dogs with mineralocorticoid deficiency, but about 25% of dogs (32% of Nova Scotia duck tolling retrievers) with hypoadrenocorticism are only deficient in glucocorticoid secretion.35,36 Dogs with a pure glucocorticoid deficiency are said to have atypical Addison's. These dogs exhibit similar clinical signs to dogs with primary hypoadrenocorticism, but they have normal electrolytes, making the diagnosis more challenging.35 Suggestive laboratory findings in cortisol-deficient dogs may include hypoglycemia, hypoalbuminemia, and the absence of a stress leukon.35 Exercise-intolerant dogs with hypoadrenocorticism will have a basal serum cortisol concentration less than 2 μg/dl, but definitive diagnosis requires confirmation with an ACTH stimulation test.37 The prognosis for recovery and function as a working dog is excellent with appropriately managed therapy.
Hypothyroidism
Hypothyroidism is common in the retriever breeds and can be associated with obesity, lethargy, and exercise intolerance caused by a decrease in metabolic rate.38 Severe chronic hypothyroidism can also cause a reversible peripheral neuropathy characterized by weakness, exercise intolerance, and hyporeflexia.39 Central nervous system atherosclerosis and thromboembolic events may be responsible for acute and chronic neurologic syndromes in hypothyroid dogs, particularly in Labrador retrievers with concurrent severe hyperlipidemia.40 Labrador retrievers with coronary artery atherosclerosis may also be predisposed to myocardial infarction.41
Dermatologic abnormalities such as alopecia, seborrhea, and pyoderma are present in over 85% of dogs with hypothyroidism, increasing the index of suspicion for this disorder.38 Laboratory testing of thyroid function, including measurement of serum total thyroxine (T4), free T4 by equilibrium dialysis (ED), and thyroid stimulating hormone (TSH), is recommended in all dogs with exercise intolerance.42 When evaluating retrievers involved in performance sports, it is important to consider that rigorous training and endurance exercise have been shown to decrease serum total T4 and free T4 in some canine athletes.43,44
Myasthenia gravis
Myasthenia gravis causes muscular weakness that typically gets worse with exercise and improves with rest.45,46 Congenital myasthenia gravis occurs rarely in dogs and is typically caused by a deficiency of acetylcholine receptors on the postsynaptic membrane. Clinical signs of weakness become apparent at 6 to 8 weeks of age, and diagnosis requires special studies to evaluate acetylcholine receptors in muscle biopsy samples of the intercostal muscles.45,46 A spontaneously resolving form of congenital myasthenia gravis has been reported in a litter of dachshunds.47
Acquired myasthenia gravis is a much more common disorder in which autoantibodies are directed against postsynaptic nicotinic acetylcholine receptors of skeletal muscle, resulting in impaired neuromuscular transmission. Golden retrievers and Labrador retrievers are commonly affected, probably reflecting the popularity of these breeds.45,46 There seems to be a bimodal age distribution, with peaks of incidence at 2 to 3 and 9 to 10 years of age.45,46
The characteristic clinical presentation of acquired myasthenia gravis in dogs is appendicular muscle weakness that worsens with exercise and improves with rest.45,46 Concurrent megaesophagus, causing regurgitation and aspiration pneumonia, is common.45,46 Physical examination of a dog with exercise intolerance caused by myasthenia gravis may be unremarkable or may reflect severe muscular weakness or aspiration pneumonia. Conscious proprioception and spinal reflexes are normal.45 Cranial nerve examination is generally normal, although in some cases repetitive stimulation of the palpebral reflex will result in muscle fatigue and an inability to blink.
Dogs with appendicular weakness due to myasthenia gravis will usually be profoundly exercise intolerant, developing weakness and collapse after only a few steps. Myasthenia gravis is not a reasonable differential diagnosis in a dog with subtle decreases in exercise tolerance or in dogs with collapse that only occurs after 10 minutes of intense activity. Myasthenia gravis is typically definitively diagnosed by demonstrating serum antibodies directed against acetylcholine receptors (Comparative Neuromuscular Laboratory, School of Medicine, University of California San Diego: http://vetneuromuscular.ucsd.edu).45,46 Response to the ultra-short-acting anticholinesterase drug edrophonium chloride (0.1 to 0.2 mg/kg intravenously in dogs) may help to establish a clinical diagnosis of myasthenia gravis while the results of confirmative antibody testing are pending. Serum antibodies to nicotinic acetylcholine receptors are detected in 98% of dogs with generalized weakness due to myasthenia gravis, but even a single dose of prednisone can result in a negative test result.46 Electrodiagnostic testing may be necessary in seronegative patients for which myasthenia gravis is still being strongly considered based on the history and clinical findings.45,46 Dogs with myasthenia gravis will have a decrement in the amplitude of muscle action potentials generated in response to repetitive nerve stimulation.
Treatment with anticholinesterase medications (e.g. pyridostigmine bromide 1-3 mg/kg orally every eight to 12 hours) and upright feedings are recommended.45,46 In patients without aspiration pneumonia, immunosuppression with low-dose prednisone and azathioprine can be recommended.45 The mortality rate from aspiration pneumonia in dogs with myasthenia gravis approaches 50%. When managed successfully, regardless of the treatment used, more than 80% of dogs experience a clinical and immunologic remission within one year of diagnosis, with an average of 6.4 months.48
Polymyositis
Polymyositis is a generalized inflammatory myopathy presumed to have an immune-mediated basis.45 Large-breed middle-aged dogs are most commonly affected.49,50 Affected dogs are usually profoundly weak, reluctant to exercise, and may have a stiff, stilted gait.50 Conscious proprioception and limb reflexes are normal. Atrophy typically occurs in the appendicular muscles and the muscles of mastication.45,49,50 Muscle pain is uncommon.50 Dysphagia, megaesophagus, and regurgitation occur in about 10% of affected dogs.49 Elevated serum creatine kinase (CK) activity is seen in most affected dogs at rest, and even more dramatic increases are common following exercise.45 The diagnosis is based on clinical signs, CK measurement, electromyography, and muscle biopsy results consistent with inflammatory muscle disease.45,49,50 Attempts should be made to rule out tick-related diseases, Toxoplasma gondii, and Neospora caninum using serology and immunohistochemistry on muscle biopsy samples.49,50 Noninfectious polymyositis is most often seen as a primary immune-mediated disorder, but it can also occur as a component of systemic lupus erythematosus, as a paraneoplastic syndrome associated with lymphoma or other tumors, or as a complication of drug administration.49,50 Treatment of primary immune-mediated polymyositis consists of immunosuppressive therapy with a good prognosis for recovery in dogs without aspiration pneumonia.45,50 Marked muscle atrophy and fibrosis due to chronically untreated polymyositis may not resolve, so it is important to confirm the diagnosis and initiate appropriate treatment early in the course of disease.
Inherited muscle disorders
A number of breed-associated inherited myopathies have been described in retrievers. These disorders cause progressive muscle weakness and exercise intolerance that becomes apparent within the first six months of life. The inherited myopathies that have been described in retrievers include dystrophin-deficient muscular dystrophy, centronuclear myopathy, and, most recently, X-linked myotubular myopathy (Table 5).
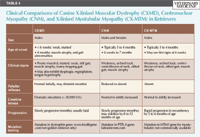
Table 5. Clinical Comparisons of Canine X-linked Muscular Dystrophy (CXMD), Centronuclear Myopathy (CNM), and X-linked Myotubular Myopathy (CX-MTM) in Retrievers
Muscular dystrophy. The muscular dystrophies are a group of inherited myopathies characterized by progressive degeneration of skeletal muscle.45,51 A condition similar to Duchenne muscular dystrophy (DMD) in people is the most common form of muscular dystrophy in dogs.51,52 Affected dogs are missing a crucial cytoskeletal protein, dystrophin, in their skeletal and cardiac muscle. The dystrophin gene is located on the X chromosome, so dystrophin-deficient muscular dystrophy is an X-linked trait that is clinically apparent in male dogs, while heterozygous females are asymptomatic carriers.51,52 Canine X-linked muscular dystrophy (CXMD) has now been reported in many breeds, but it is best described in the golden retriever and has been reported in one male Labrador retriever puppy.51,53
The severity of clinical expression of CXMD in golden retrievers is variable.54 Puppies are often stunted even before weaning. Abduction of the elbows, a bunny-hopping gait, and difficulty opening the mouth may be noted.45 With time, affected puppies develop a progressively more stilted gait; exercise intolerance; a plantigrade stance; carpal hyperextension; atrophy of the truncal, limb, and temporalis muscles; and muscle contractures.45,51,52,54 Proprioceptive positioning and spinal reflexes are normal, but spinal reflexes may be difficult to evaluate once muscle fibrosis and joint contractures occur.45,51 Tongue hypertrophy is common, and most severely affected dogs develop pharyngeal or esophageal dysfunction.45,52,54 Dilated cardiomyopathy leading to cardiac failure may occur.52 Dysphagia, lingual hypertrophy, and inspiratory stridor were prominent features in the Labrador retriever puppy with CXMD.53
CXMD should be suspected when typical clinical signs are seen in a young male retriever puppy. Serum CK activities are markedly increased as early as 1 week of age and peak at 6 to 8 weeks, just before overt clinical signs appear. After this time, CK activities plateau at about 100 times normal values, with more dramatic increases following exercise.45,52 Electromyography reveals pseudomyotonic discharges in most muscles by 10 weeks of age. Muscle biopsies reveal marked myofiber size variation, necrosis, and regeneration with multifocal myofiber mineralization. Immunocytochemical studies document the absence of the sarcolemmal protein dystrophin.51 The causative mutation for CXMD in the golden retriever has been identified, and a DNA test is commercially available (Health Gene, Toronto Ontario: www.healthgene.com/vet) for diagnosis in affected golden retrievers and to determine genetic status in carrier females. Most affected dogs become nonambulatory or develop aspiration pneumonia and are euthanized. A few dogs with less severe weakness stabilize by 6 months of age and can live functional lives as sedentary pets.45,52
Centronuclear myopathy. A muscle disorder that is inherited as an autosomal recessive trait has been reported in male and female Labrador retrievers.51,52,55 At birth, affected puppies are indistinguishable from their normal littermates, but muscular weakness, awkward gait, and decreased exercise tolerance become apparent by 2 to 6 months of age and progressively worsen.55 As the disease progresses, severely affected pups develop a low head carriage, ventroflexion of the neck, and a gait characterized by short, stilted strides.51,55,56 The hind legs are often advanced simultaneously in a synchronous, bunny-hopping fashion. Clinical signs are exacerbated by stress, excitement, and cold temperatures.51
Affected dogs exhibit pronounced atrophy of the proximal limb muscles and the muscles of mastication but no pain on muscle palpation.51,52,55 Postural reactions and proprioceptive function are normal as assessed by knuckling and hopping. Tendon reflexes (especially patellar reflexes) are reduced or absent, even in mildly affected dogs, by 1 month of age.51,55 In most dogs, the signs stabilize between 8 months and 1 year of age, so dogs that are still ambulatory at that age may remain functional as pets.52,55 Megaesophagus leading to aspiration pneumonia has been seen in a few affected dogs.55
Diagnosis should be suspected based on clinical signs, signalment, and laboratory findings. Serum CK activities are within normal limits or only mildly increased, and other routine hematologic and biochemical analyses are normal.51,52,55 Electromyography reveals spontaneous activity in the affected muscles. Histologic examination of muscle is a reliable tool for diagnosis, revealing marked variation in muscle fiber diameter with angular atrophy of both fiber types, and in some cases, a Type 1 myofiber predominance, resulting in a previous name for this condition—Type II myofiber deficiency.51,55 As the disease progresses and affected dogs mature, many myofibers display centrally placed nuclei. This feature, as well as similarities between this condition and the human muscle disorder centronuclear myopathy, prompted a change in the name of this condition to centronuclear myopathy (CNM).56 In 2003, a mutation in the gene encoding protein tyrosine phosphatase-like, member A (PTPLA) was identified as the causative genetic mutation for CNM in Labrador retrievers, and a DNA test is now commercially available (Alfort School of Veterinary Medicine, France: labradorcnm.com).56 This mutation-based test can be used to diagnose a clinically affected puppy or to screen potential breeding animals for carrier status.
X-linked myotubular myopathy. In 2008, we described a 5-month-old male Labrador retriever with rapidly progressive weakness and muscle atrophy that was clinically more severely affected than most pups with CNM.57 Muscle biopsies determined that the pup had a distinct myopathy with central mitochondrial accumulations. Multiple male littermates and half-siblings were similarly affected.57 Since that time, numerous male puppies from three different litters in western Canada have been evaluated and determined to have this same myopathy (Snead EC, Western College of Veterinary Medicine, University of Saskatchewan, Saskatoon, Saskatchewan, Canada: Unpublished data, 2009). Affected puppies first exhibit weakness and hind limb atrophy at 5 to 7 weeks of age, and this progresses to the point that they are nonambulatory or recumbent by 6 months of age.57,58 Patellar reflexes are decreased or absent, and laryngeal weakness and dysphagia occur terminally. Serum CK activity is normal or only slightly increased.57 Immunoblot analysis has revealed that myotubularin is absent in muscle extracts from affected dogs, and the mutation causing this rare X-linked myotubular myopathy has recently been identified.58
Metabolic myopathies
Metabolic myopathies can be broadly characterized as either glycolytic pathway defects or defects of oxidative metabolism.52 Although these disorders are inherited with the metabolic defect present since birth, signs of disease sometimes do not develop until adult life. Clinical findings may include weakness, muscle atrophy, exercise intolerance, and muscle cramping.
Glycogen storage diseases typically cause muscular weakness, fatigability, and hypoglycemia with exercise and may lead to the accumulation of glycogen-like material in hepatocytes and other cells.52 Exercise intolerance and hypoglycemia have been reported in curly coated retrievers with glycogen storage disease type IIIa caused by a mutation of the glycogen debranching enzyme gene.31 Affected dogs have persistently elevated serum alanine aminotransferase (ALT) and alkaline phosphatase (ALP) activities and variable increases in CK activity.31 An example of a metabolic disease that results in exercise intolerance is Type VII glycogenosis of English springer spaniels and American cocker spaniels, which is caused by a phosphofructokinase (PFK) deficiency.31,59,60 This disorder directly affects glycolysis, making it impossible for myocytes and erythrocytes to use carbohydrates for energy.60 Affected dogs have a metabolic myopathy characterized by exercise intolerance and mild increases in CK activity.31 Muscle weakness is exacerbated when alkalemia (from panting) results in intravascular hemolysis of PFK-deficient red blood cells.31,60
Pre- and post-exercise serum lactate and pyruvate concentrations should be measured in exercise-intolerant dogs suspected of having a metabolic myopathy (Comparative Neuromuscular Laboratory, School of Medicine, University of California San Diego: http://vetneuromuscular.ucsd.edu).34,51,61 Dogs with mitochondrial myopathies typically develop very high post-exercise lactate concentrations, and they may also exhibit organic aciduria.34,51,61-67 Dogs with mitochondrial defects affecting the respiratory chain will have an increased lactate-to-pyruvate ratio.51,61-63 Perhaps the best characterized mitochondrial myopathy in dogs is pyruvate dehydrogenase (PDP1) deficiency in the Clumber and Sussex spaniel.64,65 Affected dogs have profound exercise intolerance and markedly elevated resting and post-exercise plasma lactate with a normal lactate-to-pyruvate ratio.64,65
Electrodiagnostic testing and muscle biopsy should be considered in all dogs with muscle weakness and muscle atrophy suggesting a myopathy. Histologic and ultrastructural examination of skeletal muscle biopsy samples can aid in the diagnosis of a metabolic myopathy, but definitive diagnosis requires identification of the actual enzyme deficiency in tissues or detection of the causative mutation in mitochondrial or nuclear DNA.65 Metabolic screening, measurement of urinary organic acids, and DNA testing for inherited metabolic myopathies is available through the Section of Medical Genetics, School of Veterinary Medicine, University of Pennsylvania (http://research.vet.upenn.edu/penngen).
Inherited neurologic disorders
Epileptic partial seizures. Idiopathic epilepsy is a common, presumably inherited condition in Labrador retrievers and golden retrievers.68,69 Exercise, excitement, and hyperventilation can all serve as triggers for seizures in affected dogs. Most often the provoked event is a typical generalized seizure, with sudden loss of consciousness and tonic-clonic convulsions.68 Partial seizures can be more variable in their presentation, with a sudden onset of clinical signs that might include mydriasis, staring, ataxia, crawling, swaying, loss of balance, salivation, and disorientation.68-72 When these signs develop suddenly during or after exercise, the dog may be presented to a veterinarian for evaluation of exercise intolerance or, in a Labrador retriever, for a suspected diagnosis of exercise-induced collapse (EIC).73
The acute onset and rapid spontaneous recovery help to identify these episodic events as probable seizures. Also, many Labradors with exercise-provoked partial seizures will progress over months to years to having a more classic form of epilepsy with generalized seizures and seizures not necessarily associated with an exercise or excitement trigger.72 Affected dogs can be managed with standard anticonvulsant protocols using phenobarbital or potassium bromide.72
Exercise-induced collapse in Labrador retrievers. An inherited syndrome of EIC is the most common cause of exercise-induced weakness or collapse in otherwise healthy Labrador retrievers.73 Affected dogs are normal at rest and after mild or moderate exercise, but strenuous exercise in conjunction with excitement results in ataxia and rear limb weakness that sometimes progresses to collapse.73,74 Most affected dogs experience their first episode of weakness or collapse before the age of 3 years.73,75 During collapse, affected dogs are hyperthermic and they hyperventilate, but physiologic and clinicopathologic parameters are not dramatically different from normal exercise-tolerant Labrador retrievers taking part in the same exercise.74 Physical examination and neurologic examination findings are normal at rest, but patellar reflexes are absent during collapse.74 Affected dogs may experience a profound loss of balance (disequilibrium) during collapse and recovery.73,74 Most dogs gradually recover within 10 to 20 minutes after exercise is halted, with no residual clinical or clinicopathologic abnormalities, but rarely a dog will die during an episode of collapse.73,74 Muscle biopsy results are normal.74 Diagnosis is by eliminating other causes of exercise intolerance and demonstrating that the affected dog is homozygous for the causative mutation in the gene for dynamin 1 (Veterinary Diagnostic Laboratory, University of Minnesota, St. Paul MN: http://www.cvm.umn.edu/vdl/ourservices/canineneuromuscular/home.html.76
Dynamin 1 (DNM1) plays a key role in forming synaptic vesicles containing neurotransmitters and is essential for sustained synaptic transmission in the brain and spinal cord during periods of intense exercise, excitement, and perhaps hyperthermia.76 EIC is inherited as an autosomal recessive trait, and dogs with two copies of the EIC mutation are susceptible to collapse.76
Testing has revealed that the EIC mutation is common in field, show, and pet Labrador retrievers, with current data showing that 30% to 40% of Labradors are carriers and 3% to 5% of dogs in the breed are affected.75 So far, the DNM1 mutation causing EIC has only been found in Labrador retrievers, curly coated retrievers, and Chesapeake Bay retrievers.75,76 The best treatment is to avoid intensive exercise in conjunction with extreme excitement and to terminate exercise at the first sign of weakness or wobbliness.
Inherited neurodegenerative disorders
Breed-related degenerative disorders affecting the nervous system have been described in many dog breeds, including retrievers. Clinical presentations and neurologic findings reflect the nervous system tissues affected. Littermates and closely related litters are often affected, suggesting a genetic cause. Diagnosis usually requires postmortem histologic examination of nervous tissues.
Sensory ataxic neuropathy is a disorder affecting young male and female golden retrievers in Sweden.77 Affected dogs are ataxic, have profound postural reaction deficits, and have reduced or absent spinal reflexes without muscle atrophy.77,78 Signs begin before 6 months of age and are slowly progressive. Sensory nerve conduction velocities are slowed, and histologic examination reveals a chronic progressive central and peripheral sensorimotor axonopathy that is most pronounced in the proprioceptive pathways.77 A causative mutation in the mitochondrial genome was recently identified.78
Central axonopathies have been described in Labrador retrievers and in golden retrievers.79,80 Affected Labrador retrievers show pelvic limb incoordination by 5 to 6 weeks of age, progressing to hypermetria and a severe spastic ataxia affecting all four limbs.79 Most pups are nonambulatory by 3 to 5 months of age. Severe degeneration of spinal cord white matter occurs and is most pronounced in the spinocerebellar tracts in the thoracic spinal cord, medulla, cerebellar peduncles, and cerebellum.79 The axonopathy and neuronopathy reported in golden retrievers also affects pups in the first few months of life, but the signs are of severe lower motor neuron dysfunction without ataxia.80 Pelvic limb weakness progressing to tetraparesis, severe muscle atrophy, and mild hyporeflexia but normal proprioceptive positioning reflect the involvement of motor neurons in the ventral grey columns of the spinal cord.80
A number of breed-associated degenerative polyneuropathies have been reported.81 Most of these cause progressive lower motor neuron dysfunction in young dogs, with severe tetraparesis, muscle atrophy, and hyporeflexia. In some breeds, concurrent laryngeal paralysis occurs.81 Diagnosis requires electrophysiologic evaluation of nerve function and nerve biopsy.
Acquired spinal cord disease
Progressive weakness or incoordination can result in reluctance to exercise or in an altered gait. Perform a complete screening neurologic examination in all dogs with a history of exercise intolerance, and use abnormal findings to localize a lesion. Retrievers are susceptible to a wide variety of acquired spinal cord disorders including fibrocartilagenous embolism, acute and chronic intervertebral disk disease, infectious meningomyelitis, granulomatous meningoencephalitis, neoplasia, and degenerative myelopathy.13,82 Medical evaluation may include screening for infectious disease and systemic neoplasia, radiographs of the spine, and advanced imaging such as myelography, computed tomography (CT), or MRI.13,82 Genetic DNA tests for degenerative myelopathy are becoming available for a number of breeds, including Chesapeake Bay and golden retrievers (Orthopedic Foundation for Animals, Columbia, Missouri: offa.org/dnatesting).
Weakness and ataxia resulting from chronic spinal cord compression can worsen with exercise, particularly if compression exists in the cervical region where excessive motion occurs during walking and running. Intervertebral disk protrusion, articular cysts, neoplasia, or the vertebral malformations and soft tissue proliferation associated with cervical spondylomyelopathy (canine wobbler syndrome) can cause spinal cord compression.13,82
Pain may cause the reluctance to exercise in some dogs. Painful disorders of the spine may include diskospondylitis, intervertebral disk extrusion or protrusion, vertebral body neoplasia, nerve root neoplasia, polyarthritis, and meningitis.13
Acquired peripheral neuropathies
Polyneuropathies result in muscular weakness that may manifest as reluctance to exercise or exercise intolerance. These disorders typically cause generalized lower motor neuron signs that include flaccid muscle weakness or paralysis, marked muscle atrophy, decreased muscle tone, and reduced or absent reflexes.81,82 Chronic polyneuropathies can be seen in association with metabolic disorders such as hypothyroidism and diabetes mellitus.39,40,82,83 They can also occur as a paraneoplastic syndrome in dogs with insulinoma or other cancers.82
Polyneuritis that is steroid-responsive can be seen as an idiopathic immune-mediated disorder or in association with systemic lupus erythematosus. Demyelinating polyneuropathies with no known etiology and no effective treatment also occur. Suspect a polyneuropathy based on clinical findings, and confirm the diagnosis with electrodiagnostic testing and nerve biopsy. Treatment and prognosis depend on the underlying cause.82
DIAGNOSTIC EVALUATION
Diagnostic evaluation of a retriever with exercise intolerance will vary based on the description of the event or events, the physical examination findings at rest, and initial diagnostic test results. When examination at rest reveals abnormalities such as pallor, stridor, muffled heart sounds, a cardiac arrhythmia, or severe muscle atrophy, the diagnostic approach required to further investigate the underlying problem becomes obvious. When no abnormalities are identified at rest, systematically evaluate the dog to rule out metabolic, respiratory, and cardiac causes of exercise intolerance before considering muscular, neuromuscular, and neurologic disorders that require specialized testing. Once the cause of exercise intolerance has been determined, strategies for treatment or management can be developed.
Editors' note: Dr. Taylor is a patent owner of the genetic test for EIC and receives a portion of the proceeds from this test.
Kevin L. Cosford, DVM, MVetSci, DACVIM
Susan M. Taylor, DVM, DACVIM
Department of Small Animal Clinical Sciences
Western College of Veterinary Medicine
University of Saskatchewan
Saskatoon, SK S7N 5B4 Canada
REFERENCES
1. American Kennel Club News Article: Labrador Retriever holds firm in top spot on AKC's list of most popular dogs in America. http://www.akc.org/news. Jan. 21, 2009. Accessed May 27, 2009.
2. Canadian Kennel Club News: The top 20 CKC breeds for 2008. http://www.ckc.ca/en/Default.aspx?tabid=201&NewsID=275&prevID=. Feb. 4, 2009. Accessed June 28, 2009.
3. Impellizeri JA, Tetrick MA, Muir P. Effect of weight reduction on clinical signs of lameness in dogs with hip osteoarthritis. J Am Vet Med Assoc 2000;216(7):1089-1091.
4. Bach JF, Rozanski EA, Bedenice D, et al. Association of expiratory airway dysfunction with marked obesity in healthy adult dogs. Am J Vet Res 2007;68(6):670-675.
5. Johnston SA, McLaughlin RM, Budsberg SC. Nonsurgical management of osteoarthritis in dogs. Vet Clin North Am Small Anim Pract 2008;38(6):1449-1470.
6. Stull JW, Evason M, Carr AP, et al. Canine immune-mediated polyarthritis: clinical and laboratory findings in 83 cases in western Canada. Can Vet J 2008;49:1195-1203.
7. Jacques D, Cauzinille L, Bouvy B, et al. A retrospective study of 40 dogs with polyarthritis. Vet Surg 2002;31(5):428-434.
8. Rondeau MP, Walton RM, Bissett S, et al. Suppurative, nonseptic polyarthropathy in dogs. J Vet Intern Med 2005;19(5):654-662.
9. Hansson-Hamlin H, Lilliehook I. A possible systemic rheumatoid disorder in the Nova Scotia duck tolling retriever. Acta Vet Scand 2009;51(16):1-10.
10. Sharp NJH, Wheeler SJ. Lumbosacral disease. In: Small animal spinal disorders: diagnosis and surgery. 2nd ed. St. Louis, Mo.: Elsevier Mosby, 2005;181-210.
11. Suwankong N, Meij BP, Voorhout G, et al. Review and retrospective analysis of degenerative lumbosacral stenosis in 156 dogs treated by dorsal laminectomy. Vet Comp Orthop Traumatol 2008;21(23):285-293.
12. De Risio L, Thomas WB, Sharp NJ. Degenerative lumbosacral stenosis. Vet Clin North Am Small Anim Pract 2000;30(1):111-132.
13. Taylor SM. Disorders of the spinal cord. In: Nelson RW, Couto CG, eds. Small animal internal medicine. 4th ed. St. Louis, Mo.: Elsevier Mosby, 2009;1065-1091.
14. Strickland KN. Congenital heart disease. In: Tilley LP, Smith FWK, Oyama MA, Sleeper MM, eds. Manual of canine and feline cardiology. St. Louis, Mo.: Elsevier Saunders, 2008; 215-239.
15. Bonagura JD, Lehmkuhl LB. Congenital heart disease. In: Textbook of canine and feline cardiology, 2nd ed. Philadelphia, Pa.: WB Saunders, 1999;471-535.
16. Bright JM, Cali JV. Clinical usefulness of cardiac event recording in dogs and cats examined because of syncope, episodic collapse or intermittent weakness: 60 cases (1997-1999). J Am Vet Med Assoc 2000;216(7):1110-1114.
17. Schrope DP, Kelch WJ. Signalment, clinical signs, and prognostic indicators associated with high-grade second- or third-degree atrioventricular block in dogs: 124 cases (January 1, 1997-December 31, 1997). J Am Vet Med Assoc 2006;228(11):1710-1717.
18. Kraus MS, Calvert CA. Syncope. In: Bonagura JD, Twedt DC, eds. Current veterinary therapy XIV. St. Louis, Mo.: Elsevier Saunders, 2009;709-712.
19. Shaw SP, Rush JE. Canine pericardial effusion: diagnosis, treatment and prognosis. Compend Contin Educ Vet 2007;29(7):405-411.
20. Shaw SP, Rush JE. Canine pericardial effusion: pathophysiology and cause. Compend Contin Educ Vet 2007;29(7):400-403.
21. McKiernan BC. Sounds and signs: localizing respiratory diseases, in Proceedings. 76th Annual Western Vet Conf 2004.
22. Tobias KM, Millard RP. Laryngeal paralysis in dogs. Compend Cont Educ Vet 2009;31(5):212-219.
23. MacPhail CM, Monnet E. Outcome of and postoperative complications in dogs undergoing surgical treatment of laryngeal paralysis: 140 cases (1985-1998). J Am Vet Med Assoc 2001;218:1949-1956.
24. Snelling SR, Edwards GA. A retrospective study of unilateral arytenoid lateralisation in the treatment of laryngeal paralysis in 100 dogs (1992-2000). Aust Vet J 2003;81:464-468.
25. Jeffery ND, Talbot CE, Smith PM, et al. Acquired idiopathic laryngeal paralysis as a prominent feature of generalised neuromuscular disease in 39 dogs. Vet Rec 2006;158:17-21.
26. Hammel SP, Hottinger HA, Novo RE. Postoperative results of unilateral arytenoid lateralization for treatment of laryngeal paralysis in dogs: 39 cases (1996-2002). J Am Vet Med Assoc 2006;228:1215-1220.
27. Morrison WB. Pallor. In: Ettinger SJ, Feldman EC, eds. Textbook of veterinary internal medicine. 6th ed. St. Louis, Mo.: Elsevier Saunders, 2005;211-215.
28. Johnson RK, Atkins CE. Non-neoplastic causes of canine hypoglycemia. In: RW Kirk, ed. Current veterinary therapy VII. Philadelphia, Pa.: WB Saunders, 1980;1023-1027.
29. Feldman EC, Nelson RW. Beta-cell neoplasia: insulinoma. In: Canine and feline endocrinology and reproduction. 3rd ed. St. Louis, Mo.: Elsevier Saunders, 2004;616-634.
30. Dunayer EK. Hypoglycemia following canine ingestion of xylitol-containing gum. Vet Hum Toxicol 2004;46(2):87-88.
31. Gregory BL, Shelton GD, Bali DS, et al. Glycogen storage disease type IIIa in curly-coated retrievers. J Vet Intern Med 2007;21(1):40-46.
32. Syme HM, Scott-Moncrieff JC. Chronic hypoglycaemia in a hunting dog due to secondary hypoadrenocorticism. J Small Anim Pract 1998;39:348-351.
33. Angle CT, Wakshlag JJ, Gillette RL, et al. Hematologic, serum biochemical, and cortisol changes associated with anticipation of exercise and short duration high-intensity exercise in sled dogs. Vet Clin Pathol 2009;38(3):370-374.
34. Matwichuk CL, Taylor SM, Shmon CL, et al. Changes in rectal temperature and hematologic, biochemical, blood gas and acid-base values in healthy Labrador retrievers before and after strenuous exercise. Am J Vet Res 1999;60(1):88-92.
35. Thompson AL, Scott-Moncrieff JC, Anderson JD. Comparison of classic hypoadrenocorticism with glucocorticoid-deficient hypoadrenocorticism in dogs: 46 cases (1985-2005). J Am Vet Med Assoc 230(8):1190-1194.
36. Hughes AM, Nelson RW, Famula TR, et al. Clinical features and heritability of hypoadrenocorticism in Nova Scotia duck tolling retrievers: 25 cases (1994-2006). J Am Vet Med Assoc 2007;231(3):407-412.
37. Lennon EM, Boyle TE, Grace Hutchins R, et al. Use of basal serum or plasma cortisol concentrations to rule out a diagnosis of hypoadrenocorticism in dogs: 123 cases (2000-2005). J Am Vet Med Assoc 2007;231(3):413-416.
38. Panciera DL. Conditions associated with hypothyroidism. Vet Clin North Am Small Anim Pract 2001;31(5):935-947.
39. Jaggy A, Oliver JE, Ferguson DC, et al. Neurological manifestations of hypothyroidism: a retrospective study of 29 dogs. J Vet Intern Med 1994;8:328-336.
40. Vitale CL, Olby NJ. Neurologic dysfunction in hypothyroid, hyperlipidemic Labrador retrievers. J Vet Intern Med 2007;21(6):1316-1322.
41. Liu S, Tilley PL, Tappe JP, et al. Clinical and pathologic findings in dogs with atherosclerosis: 21 cases (1970-1983). J Am Vet Med Assoc 1986;189:227-232.
42. Scott-Moncrieff JCR, Guptill-Yoran L. Hypothyroidism. In: Ettinger SJ, Feldman EC, eds. Textbook of veterinary internal medicine. 6th ed. St. Louis, Mo.: Elsevier Saunders, 2005;1535-1544.
43. Hill RC, Lewis DD, Beale KM, et al. Effects of racing and training on serum thyroid hormone concentrations in racing greyhounds. Am J Vet Res 2001;62(12):1969-1972.
44. Evason MD, Carr AP, Taylor SM, et al. Alterations in thyroid hormone concentrations in healthy sled dogs before and after athletic conditioning. Am J Vet Res 2004;65(3):333-337.
45. Taylor SM. Selected disorders of muscle and the neuromuscular junction. Vet Clin North Am Small Anim Pract 2000;30(1):59-75.
46. Shelton GD. Myasthenia gravis and disorders of neuromuscular transmission. Vet Clin North Am Small Anim Pract 2002;32(1):189-206.
47. Dickinson PJ, Sturges BK, Shelton GD, et al. Congenital myasthenia gravis in smooth-haired miniature dachshund dogs. J Vet Intern Med 2005;19:920-923.
48. Shelton GD, Lindstrom JM. Spontaneous remission in canine myasthenia gravis: implications for assessing human MG therapies. Neurology 2001;57(11):2139-2141.
49. Evans J, Levesque D, Shelton GD. Canine inflammatory myopathies: a clinicopathologic review of 200 cases. J Vet Intern Med 2004;18:679-691.
50. Podell M. Inflammatory myopathies. Vet Clin North Am Small Anim Pract 2002;32(1):147-167.
51. Shelton GD, Engvall E. Muscular dystrophies and other inherited myopathies. Vet Clin North Am Small Anim Pract 2002;32(1):103-124.
52. Blot S. Disorders of the skeletal muscle — canine. In: Ettinger SJ, Feldman EC, eds. Textbook of veterinary internal medicine. 6th ed. St. Louis, Mo.: Elsevier Saunders, 2005;901-905.
53. Bergman RL, Inzana KD, Monroe WE, et al. Dystrophin deficient muscular dystrophy in a Labrador retriever. J Am Anim Hosp Assoc 2002;38(3):255-261.
54. Ambrosio CE, Fadel L, Gaiad TP, et al. Identification of three distinguishable phenotypes in golden retriever muscular dystrophy. Gen Mol Res 2009;8(2):389-396.
55. Klopp LS, Smith BF. Autosomal recessive muscular dystrophy in Labrador retrievers. Compend Contin Educ Vet 2000;22(2):121-130.
56. Pele M, Tiret L, Kessler JL, et al. SINE exonic insertion in the PTPLA gene leads to multiple splicing defects and segregates with the autosomal recessive centronuclear myopathy in dogs. Hum Mol Genet 2005;14(11):1417-1427.
57. Cosford KL, Taylor SM, Thompson L, et al. A possible new inherited myopathy in a young Labrador retriever. Can Vet J 2008;49:393-397.
58. Shelton GD, Snead E, Bohm J, et al. A missense mutation in MTM1 gene causes X-linked myotubular myopathy in Labrador retrievers (abst), in Proceedings. Am Coll Vet Intern Med Forum, 2009.
59. Skibild E, Dahlgaard K, Rajpurohit Y, et al. Haemolytic anemia and exercise intolerance due to phosphofructokinase deficiency in related springer spaniels. J Small Anim Pract 2001;42(6):298-300.
60. Harvey JW. Pathogenesis, laboratory diagnosis and clinical implications of erythrocyte enzyme deficiencies in dogs, cats and horses. Vet Clin Pathol 2006;35(2):144-156.
61. Shelton GD, Nyhan WL, Kass PH, et al. Analysis of organic acids, amino acids and carnitine in dogs with lipid storage myopathy. Muscle Nerve 1998;21:1202-1205.
62. Breitschwerdt EB, Kornegay JN, Wheeler SJ, et al. Episodic weakness associated with exertional lactic acidosis and myopathy in Old English Sheepdog littermates. J Am Vet Med Assoc 1992;201:731-735.
63. Olby NJ, Chan KK, Targett MP et al. Suspected mitochondrial myopathy in a Jack Russell terrier. J Small Anim Pract 1997;38:213-216.
64. Abramson CJ, Platt SR, Shelton GD. Pyruvate dehydrogenase deficiency in a Sussex spaniel. J Small Anim Pract 2004;45(3):162-165.
65. Cameron JM, Maj MC, Levandovskiy V, et al. Identification of a canine model of pyruvate dehydrogenase phosphatase 1 deficiency. Mol Genet Metab 2007;90(1):15-23.
66. Tauro A, Talbot CE, Pratt JNJ, Boydell IP. Suspected mitochondrial myopathy in a Springer spaniel. Vet Rec 2008;163(13):396-397.
67. Paciello O, Maioling P, Fatone G, et al. Mitochondrial myopathy in a German Shepherd dog. Vet Pathol 2003;40:507-511.
68. Jaggy A, Faissler D, Gaillard C, et al. Genetic aspects of idiopathic epilepsy in Labrador retrievers. J Small Anim Pract 1998;39:275-280.
69. Berendt M, Gredal H, Gam Pedersen L, et al. A cross-sectional study of epilepsy in Danish Labrador retrievers: prevalence and selected risk factors. J Vet Intern Med 2002;16:262-268.
70. Berendt M, Gullov CH, Christensen SLK, et al. Prevalence and characteristics of epilepsy in the Belgian shepherd variants Groenendael and Tervueren born in Denmark 1995-2004. Acta Vet Scand 2008;50(1):51,1-7.
71. Patterson EE, Da Y, Mickelson JR, et al. Clinical characteristics and inheritance of idiopathic epilepsy in Vizslas. J Vet Intern Med 2003;17(3):319-325.
72. Heynold Y, Faissler D, Steffen F, et al. Clinical, epidemiological and treatment results of idiopathic epilepsy in 54 Labrador retrievers: a long term study. J Small Anim Pract 1997;38:7-14.
73. Taylor SM, Shmon CL, Shelton GD, et al. Exercise induced collapse of Labrador retrievers: clinical description of the syndrome and preliminary investigation of heritability. J Am Anim Hosp Assoc 2008;44:295-301
74. Taylor SM, Shmon CL, Adams VJ, et al. Evaluations of Labrador retrievers with exercise induced collapse, including response to a standardized strenuous exercise protocol. J Am Anim Hosp Assoc 2009;45:3-13.
75. Minor KM, Patterson EE, Gross SD, et al. Frequency of the canine exercise induced collapse (EIC) gene in diverse breeds (abst). J Vet Intern Med 2009;23(3):741.
76. Patterson EE, Minor KM, Tchernatynskaia AV, et al. A canine dynamin 1 mutation is highly associated with the syndrome of exercise-induced collapse. Nature Genetics 2008;40(10):1235-1239.
77. Jaderlund KH, Orvind E, Johnsson E, et al. A neurologic syndrome in golden retrievers presenting as a sensory ataxic neuropathy. J Vet Intern Med 2007;21:1307-1315.
78. Baranowska I, Jaderlund KH, Nennesmo I, et al. Sensory ataxic neuropathy in golden retriever dogs is caused by a deletion in the mitochondrial tRNATyr Gene. PLoS Genet 2009;5(5):1-9.
79. de Lahunta A, Ingram JT, Cummings JF, et al. Labrador retriever central axonopathy. Prog Vet Neurol 1994;5:117-122.
80. da Costa RC, Parent JM, Poma R, et al. Multisystem axonopathy and neuronopathy in golden retriever dogs. J Vet Intern Med 2009;23:935-939.
81. Coates JR, O'Brien DP. Inherited peripheral neuropathies in dogs and cats. Vet Clin North Am Small Anim Pract 2004;34:1361-1401.
82. Olby NJ. Tetraparesis. In: Platt SR, Olby NJ, eds. BSAVA manual of canine and feline neurology. 3rd ed. Hoboken, N.J.: Wiley, 2004-;214-236.
83. Morgan MJ, Vite CH, Radhakrishnan A, et al. Clinical peripheral neuropathy associated with diabetes mellitus in three dogs. Can Vet J 2008;49(6):583-586.