Fluoroquinolones and pyoderma: Using the best drug at the right dosage (Sponsored by Pfizer)
Fluoroquinolone antibiotics represent one of the greatest developments in antibiotic therapy in the last fifty years. However, with their tremendous benefit comes great risk.
Fluoroquinolone antibiotics represent one of the greatest developments in antibiotic therapy in the last fifty years. However, with their tremendous benefit comes great risk.1,2
Fluoroquinolones are one of the most effective groups of antibiotics developed to date because of their unique and potent mechanism of action. They specifically target DNA gyrase molecules in bacterial cells, making it impossible for those cells to reproduce.3,5 This targeted effect makes fluoroquinolones highly effective against susceptible bacteria when used at appropriate doses for the proper duration of therapy.
Fluoroquinolones are widely used based on their extended-spectrum of activity, ease of administration, and low toxicity.6 These drugs are well-absorbed upon oral administration and are widely distributed into most tissues.6,7 Fluoroquinolones accumulate within white blood cells, increasing in concentration at the target tissue as long as active inflammation is present.6,7 Because of the extended half-life of these drugs and the way they persistently suppress bacterial growth after dosing (the postantibiotic effect), fluoroquinolones can be administered once daily, which improves compliance.
Fluoroquinolone antibiotics kill bacteria rapidly if used at appropriate concentrations in the target tissue based on the organism's minimum inhibitory concentration (MIC).6-8 This class of antibiotics is concentration-dependent, so the peak drug concentration after administration in the target tissue (skin, kidney, urine) is the most important dosing factor. To achieve high bactericidal concentrations, it is extremely important to select an appropriate dose or resistance may develop.
Studies have shown that the inhibitory quotient and the area under the inhibition curve (AUIC) are the most important factors in predicting fluoroquinolone efficacy while minimizing the development of resistant bacteria. These parameters are calculated as:
Inhibitory quotient = Cmax ÷ MIC90
AUIC = AUC ÷ MIC90
Ideally these calculations should include the Cmax at the target tissue and the MIC for the pathogen being treated. Often the Cmax at the target tissue is not known, and the plasma Cmax, where drug concentrations are lower than many target tissues, is used. For the fluoroquinolone to be effective, the inhibitory quotient should be greater than or equal to eight, the AUIC should be greater than or equal to 125, and the dose should be adjusted to achieve these levels. These values were derived from in vitro studies or studies involving neutropenic rats or critically ill humans and do not take into account the effect of a functioning immune system.
The mutant selection window is a new theory on dose selection to minimize the development of resistance. It proposes that a drug concentration range (selection window) exists for which mutation of the pathogen is promoted. Current dosing practices for fluoroquinolones tend to place drug concentrations in some target tissues inside this window. By identifying and avoiding this window, the amplification of selecting for resistant pathogens can be slowed. The mutant prevention concentration (MPC) is the concentration of an antibiotic that inhibits the growth of the least susceptible subpopulation of a pathogen when more than 1010 cells are tested. Above this concentration, a bacteria must acquire two concurrent resistance mutations for growth, which rarely occurs. The lower boundary of the appropriate selection window is approximated by the MIC90 and the upper boundary by the MPC. The selection window hypothesis has not been extensively tested in human or veterinary medicine, and data are still lacking.
For Staphylococcus intermedius commonly associated with canine pyoderma, MIC90s for fluoroquinolones are relatively low. However, the skin is one of the most difficult tissues in which to achieve drug concentrations, and a higher dosage should be considered in treating pyodermas. In general, the higher end of the dose range (approximately the MPC) is used in treating pyodermas in a referral dermatology practice to compensate for the decreasing tissue concentrations as the pyoderma resolves and inflammation diminishes (Table 1).9

Table 1: Fluoroquinolone Dosages for Treating Recurrent Pyoderma
Resistance risks
Fluoroquinolones are often selected to treat cases of resistant bacterial infection because of their perceived high potency.2 When dosed correctly and administered to immunocompetent animals, fluoroquinolone antibiotics have extremely good efficacy and kill bacteria quickly. But if administered at suboptimal doses for short durations or concurrently with high doses of immunosuppressive drugs, such as prednisone, the potential for the bacteria to develop resistance to multiple antibiotics increases.10,12
Resistance to fluoroquinolones occurs when bacteria are repeatedly exposed to suboptimal antibiotic doses, which select for resistant isolates in the bacterial population.11 As long as high doses are used for a long enough time to effectively kill the bacteria, fluoroquinolone resistance is unlikely to develop. Unfortunately, fluoroquinolone therapyis expensive when dosed appropriately. This may cause practitioners to prescribe suboptimal doses for shorter than ideal durations, increasing the selection of resistant infections. If a patient fails to respond initially or completely, it is essential that lesions be cultured for aerobic bacteria and an antibiotic sensitivity panel be conducted to appropriately modify the antibiotic selection and dosing protocol.
When exposed repeatedly to low doses of fluoroquinolones, most bacterial organisms change gradually as chromosomally mediated mutations eventually lead to resistance.7,13 These mutations take place in bacterial genes that code for subunits of DNA gyrase, topoisomerase IV, or expression of multiple-drug-resistant efflux pumps.3,5 Mutations in DNA gyrase are thought to be most significant in gram-negative bacteria, while topoisomerase IV is the primary target in gram-positive bacteria.3
This resistance proceeds in a two-step sequence. The first step mutation provides the bacteria with only low-level resistance but makes the second step mutation more likely.14,16 Once the second step mutation occurs, the bacteria are fully resistant not only to the specific antibiotic but very likely to the entire class of fluoroquinolones. Previous exposure to fluoroquinolones, especially if administered at suboptimal doses, greatly increases the likelihood that the first step mutation has occurred and progression to the second step is inevitable.
Additionally, both Pseudomonas aeruginosa and Staphylococcus aureus are able to express multiple drug efflux pumps, which play a role in antibiotic resistance.4,5 Exposure to suboptimal doses of fluoroquinolones can select for mutations in the expression of these efflux pumps in as little as four days.4 This rapid upregulation of the efflux pumps allows bacterial cells to efficiently excrete the antibiotic before they are affected by the drug. Resistance seems to be mediated by repeated exposure to suboptimal doses of the antibiotics rather than a single mutation event.14 This suggests that fluoroquinolone resistance is unlikely, as long as high doses are used for prolonged durations.
In addition, the individual drugs within the fluoroquinolone group exhibit different binding affinities for the bacterial DNA gyrase molecule, resulting in differences in potency and efficacy.8 These factors can be compensated for if the drug reaches high enough tissue concentrations to completely overwhelm not only the organism's efflux pumps, but also the discrepancies in binding affinity for the specific molecule and binding sites.
Recent evidence suggests that fluoroquinolones may upregulate the genes responsible for methicillin resistance.17,18 Methicillin resistance is controlled by a mec-A gene cassette that regulates the bacterial genes responsible for resistance to penicillins and cephalosporins. Therefore, exposure to suboptimal doses of fluoroquinolones increases the risk of not only fluoroquinolone resistance but also methicillin resistance. Clinically, this means that the misuse of fluoroquinolones can potentially cause resistance in multiple antibiotic classes simultaneously.
Guidelines for treating pyoderma
When an underlying skin disease causes the skin's natural antimicrobial defenses (e.g., sebum, pH, epidermal turnover) to malfunction, secondary bacterial infections often result. Allergies associated with environmental factors, certain foods, and fleas, along with endocrinopathies such as hypothyroidism and Cushing's disease, are the most common diseases linked to secondary bacterial pyoderma. Other possible underlying dermatoses include autoimmune skin disease and keratinization defects. Clinicians should use an aggressive diagnostic workup to explore a patient's endocrine status and identify allergic disease. Cutaneous biopsies are often useful in determining whether a patient is experiencing cutaneous changes typical of an allergy, endocrine disease, autoimmune skin disease, or a keratinization defect.
When the underlying dermatosis is successfully controlled, the natural antimicrobial functionality of the skin returns and the recurrent nature of the infection is eliminated. If primary underlying dermatoses cannot be identified or controlled, patients will likely continue to develop secondary bacterial infections, which often lead to multiple-drug-resistant staphylococcal pyoderma.
Fluoroquinolone antibiotics offer a unique opportunity to treat pyoderma successfully if certain minimum requirements are met. As discussed previously, their effectiveness is extremely dependent on optimal dosing. In order to achieve the benefit of fluoroquinolone antibiotics, high concentrations are required to overwhelm the bacteria's DNA gyrase system and completely inhibit organism replication.
In general, fluoroquinolones should not be used as first-line antibiotics for routine pyoderma (Table 2). Since these infections are caused by an underlying cutaneous disorder that has altered the normal skin's defense mechanism, they can often be successfully treated with cephalosporins while simultaneously identifying and correcting the underlying dermatosis.
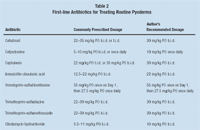
Table 2: First-line Antibiotics for Treating Routine Pyoderma
Fluoroquinolones become the drug of choice for known resistant infections. Multiple-drug resistance has historically been rare in our canine patients; however, the incidence is increasing. Recently, a new and potentially more aggressive species of Staphylococcus, Staphylococcus schleiferi, was isolated from dogs with recurrent pyoderma and otitis.19 S. schleiferi seems to more readily develop multiple-drug resistance when exposed to frequent antibiotic treatments, as is typical in dogs with allergic or endocrine skin disease.19 Additionally, human methicillin-resistant S. aureus infections seem to be spreading into our canine patients.20,24 These unique bacterial species and resistant infections should be treated aggressively with high-dose fluoroquinolone treatment alone or in combination with other systemic antibiotics.
Fluoroquinolone antibiotics should be used to treat nonresponsive bacterial infections after culture and sensitivity testing indicate sensitivity to this class of drugs. The importance of using high doses of fluoroquinolones cannot be overemphasized. If the proper dose is selected and administered for one week past complete clinical resolution (a minimum of three weeks), the aggressive, multiple-drug-resistant infection should respond. However, if suboptimal doses are administered, the potential for multiple-class antibiotic resistance is extremely high.
Conclusion
In practical terms, the beneficial efficacy of fluoroquinolones is completely dependent on achieving a high drug concentration within the tissue. It is also important to determine the target bacterial organism so that the fluoroquinolone can reach optimal concentration in the tissue for the bacteria's expected MIC level—or, more importantly, the concentration needed to prevent mutation, given the specific infection site and organism.
However, if dosed at optimal levels (high doses with frequent and prolonged administration intervals), fluoroquinolones remain the most effective and lethal groups of antibiotics for bacterial infections in numerous organ systems.
References
1. Limoncu MH, Ermertcan S, Cetin CB, et al. Emergence of phenotypic resistance to ciprofloxacin and levofloxacin in methicillin-resistant and methicillin-sensitive Staphylococcus aureus strains. Int J Antimicrob Agents 2003;21:420-424.
2. Van Bambeke F, Michot JM, Van Eldere J, et al. Quinolones in 2005: An update. Clin Microbiol Infect 2005;11:256-280.
3. Lloyd DH, Lamport AI, Noble, WC, et al. Fluoroqinolone resistance in Staphylococcusintermedius. Vet Dermatol 1999;10:249-251.
4. Hooper DC. Emerging mechanisms of fluoroquinolone resistance. Emerging Infect Dis 2001;7:337-341.
5. Le Thomas I, Couetdic G, Clermont O, et al. In vivo selection of a target/efflux double mutant of Pseudomonasaeruginosa by ciprofloxacin therapy. J Antimicrob Chemother 2001;48:553-555.
6. Walker, RD. Fluoroquinolones. In: Prescott JF, Baggot JD, Walker RD, eds. Antimicrobial therapy in veterinary medicine. 3rd ed. Ames, Iowa: Iowa State University Press, 2000;315-338.
7. Boothe DM. Antimicrobial drugs. Small animal clinical pharmacology and therapeutics. Philadelphia, Pa: WB Saunders Co, 2001;150-173.
8. Frazier DL, Thompson L, Trettien A, et al. Comparison of fluoroquinolone pharmacokinetic parameters after treatment with marbofloxacin, enrofloxacin, and difloxacin in dogs. J Vet Pharmacol Ther 2000;23:293-302.
9. DeManuelle TC, Ihrke PJ, Brandt CM, et al. Determination of skin concentrations of enrofloxacin in dogs with pyoderma. Am J Vet Res 1998;59:1599-1604.
10. Papich MG, White SD. Fluoroquinolone use and antibiotic resistance, in Proceedings. 17th Annu Meet Am Acad Vet Dermatol/Am Coll Vet Dermatol 2002; 203-204.
11. Pirro F, Edingloh M, Schmeer N. Bacteridical and inhibitory activity of enrofloxacin and other fluoroquinolones in small animal pathogens, in Proceedings. 3rd Int Vet Symp on Baytril 1999;21:19-25.
12. Blondeau JM, Hansen G, Metzler K, et al. The role of PK/PD parameters to avoid selection and increase of resistance: Mutant prevention concentration. J Chemother 2004;16(suppl 3):1-9.
13. Prescott JF. Antimicrobial drug resistance and its epidemiology. In: Prescott JF, Baggot JD, Walker RD, eds. Antimicrobial therapy in veterinary medicine. 3rd ed. Ames, Iowa: Iowa State University Press, 2000;27-49.
14. Neu HC. Bacterial resistance to fluoroquinolones. Rev Infect Dis 1988;10(suppl 1):S57-S63.
15. McKellar QA, Sanchez Bruni SF, Jones DG, et al. Pharmacokinetic/pharmacodynamic relationships of antimicrobial drugs used in veterinary medicine. J Vet Pharmacol Ther 2004;27:503-514.
16. Fuller JD, Low DE. A review of Streptococcuspneumoniae infection treatment failures associated with fluoroquinolone resistance. Clin Infect Dis 2005;41:118-121.
17. Nseir S, DePompeo C, Soubrier S, et al. First-generation fluoroquinolone use and subsequent emergence of multiple drug-resistant bacteria in the intensive care unit. Crit Care Med 2005; 33:283-289.
18. Sierra JM, Marco F, Ruiz J, et al. Correlation between the activity of different fluoroquinolones and the presence of mechanisms of quinolone resistance in epidemiologically related and unrelated strains of methicillin-susceptible and -resistant Staphylococcusaureus. Clin Microbiol Infect 2002;8:781-790.
19. Frank LA, Kania SA, Hnilica KA, et al. Isolation of Staphylococcusschleiferi from dogs with pyoderma. J Am Vet Med Assoc 2003;222:451-454.
20. Weese JS. Methicillin-resistant Staphylococcusaureus: An emerging pathogen in small animals. J Am Anim Hosp Assoc 2005;41:150-157.
21. Manian FA. Asymptomatic nasal carriage of mupirocin-resistant, methicillin-resistant Staphylococcusaureus (MRSA) in a pet dog associated with MRSA infection in household contacts. Clin Infect Dis 2003;36:e26-e28.
22. Duquette RA, Nuttall TJ. Methicillin-resistant Staphylococcusaureus in dogs and cats: An emerging problem? J Small Anim Pract 2004;45:591-597.
23. Shaw SE. Dogs, cats and methicillin-resistant Staphylococcusaureus. J Small Anim Pract 2004;45:587-588.
24. Van Duijkeren E, Van Laar P, Houwers DJ. Methicillin-resistant staphylococci isolated from animals. Vet Microbiol 2004;103:91-97.