Gastric dilatation volvulus syndrome (Proceedings)
Gastric dilatation volvulus syndrome is an acute medical and surgical condition due to several pathophysiological effects occurring secondary to gastric distension and mal-positioning. It occurs most commonly in large, deep chested dogs.
Gastric dilatation volvulus syndrome is an acute medical and surgical condition due to several pathophysiological effects occurring secondary to gastric distension and mal-positioning. It occurs most commonly in large, deep chested dogs. Gastric dilatation volvulus should be differentiated from food engorgement. Food engorgement results from the over consumption of food and can result in severe dilation of the stomach.
Chronic gastric dilatation volvulus has been recognized at several occasions in dogs. The diagnosis is more subtle because the clinical signs are not as dramatic as for an acute gastric dilatation volvulus. Dogs with chronic dilatation volvulus have an history of chronic vomiting, flatulence, and weight loss.
Aerophagia is the most likely source of gas accumulation. Bacterial fermentation of carbohydrate, gas production from acid-bicarbonate reactions may contribute to gas accumulation. The fluid component of gastric contents is a combination of ingesta, gastric secretions, and transudate from venous obstruction.
The mechanism of gastric outflow obstruction is unknown. As gastric dilatation progresses, the normal means of relief, such as eructation, vomiting, or pyloric emptying fail to occur. Pyloric function seems to be normal in dog treated for gastric dilatation volvulus. Acute gastric dilatation volvulus has been recognized for many year in dogs however its exact cause is still not clearly understood in dogs. Only risk factors have been identified. Increased gastrin level, decreased stomach motility and delayed gastric emptying have been mentioned as a risk factor but never been demonstrated. Diet, the amount of food ingested, the frequency of feeding, behavior (fast eating style), exercise and stress after a meal are contributing factors for the development of gastric dilatation volvulus. Large breed dogs, dogs with large thoracic depth-to-width ratio, underweight dogs and older animals are at higher risk to develop a gastric dilatation volvulus. Dogs with an happy personality might be at less risk. Removal of a large spleen has been associated with the development of gastric dilatation volvulus.
A clockwise or a counter-clockwise rotation of the stomach is possible. The most common rotation is clockwise. Displacement of the pylorus occurs from right toward ventral midline, passing over the gastric fundus and body to an area along the left abdominal wall close to the lower esophageal sphincter. At the same time the fundus goes in a ventral direction toward the right abdominal wall. Because of the attachment of the omentum to the greater curvature of the stomach, the omentum covers the stomach after volvulus. The displacement of the spleen may vary from the degree of volvulus. The spleen is usually engorged or can undergo torsion on its own pedicle. Thrombosis of the splenic artery can also occur. Most commonly 180° of rotation is seen but 360° rotation is possible. Counterclockwise rotation is not common. In this type of rotation the pylorus and antrum displace dorsally along the right abdominal wall. Fundus and body of the stomach go through minimal ventral displacement, and the omentum does not cover the stomach. The degree of rotation is limited to 90° .
Gastric dilatation causes compression of the caudal vena cava and portal vein. Sequestration of blood in the spleen, kidney, and gastrointestinal tract occurs. Compression of the caudal vena cava and portal vein induces a decreased venous return resulting in hypovolemic shock. Hypotension and venous stasis result in cellular hypoxia and anaerobic metabolism. Focal myocardial ischemia and hypoxia will reduce contractility and induce arrhythmias. Vascular stasis, hypoxia and acidosis can predispose to the development of disseminated intravascular coagulopathy. Respiratory dysfunction results from decreased pulmonary compliance and mechanical restriction of diaphragmatic movement by a dilated stomach. Tissue hypoxia results from decreased cardiac output and respiratory impairment. Increased gastric intraluminal pressure, portal hypertension, and venous stasis with thrombosis result in gastric mucosa stasis, hypoxia, and edema. Gastric wall necrosis can then develop in the fundus along the greater curvature Vascular wall disruption results in mucosal hemorrhage. Avulsion of branches from the short gastric arteries contributes to the blood loss, hypovolemia, and restriction of blood flow to the stomach.
Clinical Signs
Dogs with gastric dilatation volvulus are presented with progressive distension and tympanic cranial abdomen. They are restlessness, retching, and hypersalivating. Weak peripheral pulse, pale mucous membrane, increased heart rate, prolonged capillary time, and tachypnea are present too. Abdominal pain is usually not present on palpation.
Dogs with a gastric dilatation volvulus are presented with a weak femoral pulse, increased heart rate, pale mucous membrane, decreased capillary refill time, and tachypnea. Hypovolemic shock can be present at the time of presentation.
Diagnosis
Very often the diagnosis is made from the history, the signalment and the physical examination. Radiographic evaluation is rarely required for the diagnosis of gastric dilatation volvulus.
Dogs are usually presented for unproductive vomiting, retching and hypersalivation. The abdomen is distended and as the severity increases the animal can become weak, lateral recumbent with tachypnea.
Signalment
Gastric dilatation volvulus is most commonly seen in large and giant breed dogs. It also can be seen in small dogs and cats. Dogs from 10 month to 14 years old have been diagnosed with gastric dilatation volvulus. No sex predilection have been demonstrated.
Physical Examination
On physical examination is classic to find a large distended abdomen. On percussion, a tympanic sound is produced. Abdominal palpation could be uncomfortable. Animals hypersalivate and are retching.
Gastric dilatation volvulus induces different degree of shock that needs to be recognized during the evaluation of the patient. First, the animals are presented with clinical signs similar to hypovolemic shock because most of their blood volume is restricted in the caudal vena cava and the portal vein. Therefore, the animals are going to be tachycardic and tachypnic with a normal femoral pulse, a slow capillary refill time, pale mucous membrane, and cold extremities. With progression of the syndrome the patients are going to become in endotoxemic shock with tachycardia, tachypnea, weak femoral pulse, injected mucous membrane, fever, slow capillary refill time. Finally the patients are going to decompensate with severe hypotension, bradychardia, hypothermia, white mucous membranes and cold extremities.
The severity of the presentation is indicator of survival for the patients. Dogs that are presented bright and alert have better prognosis than dogs that lateral recumbent.
Radiographs
Radiographs help to differentiate between gastric dilation and gastric dilation volvulus syndrome. Radiographs if necessary for the diagnosis are not performed until the patient is stable. Since the pylorus is displaced on the left side of the abdominal cavity in a dorso-cranial position to the fundus a right lateral recumbency radiographs are required to be able to have a double bubble image. The two bubbles are due to the accumulation of air in the pylorus and the fundus. Free gas is present in the abdomen when the stomach has ruptured.
If only gastric dilatation is present without volvulus, radiographs show a dilated stomach with dilated loop of jejunum.
Treatment
Emergency Treatment
Emergency medical treatment of the hypovolemic shock and gastric decompression is required before surgical treatment for gastric dilatation volvulus. Venous catheters of the largest size possible are placed in the cephalic or jugular vein to deliver shock dose of intravenous fluid. Isotonic fluids are delivered at an initial rate of 90 ml/kg during the first 30 to 60 mn. The rate and volume of fluids administered can be adjusted according to assessment of several clinical parameters: heart rate, pulse, mucous membrane, capillary refill time and measurement of central venous pressure. Colloids at the dose of 4 ml/kg are recommended during hypovolemic shock treatment with sodium chloride or Normosol. Hypertonic saline solution also has a positive inotrope effect. Corticosteroid may have potential beneficial effect in case of endotoxic shock with stabilization of vascular and lysosomal membrane. Free radical scavengers (allopurinol) also have a potential indication before gastric derotation to prevent reperfusion injury. Blood gas and electrolyte evaluations are required before acid-base and electrolyte imbalances corrections are attempted. A dog presented with a gastric dilatation volvulus can be either alkalotic or acidotic, hypokalemic or normokalemic.
Gastric decompression is attempted first with an orogastric tube after initiation of fluid therapy. Patients' compliance, the amount of gastric distension, and the degree of gastric volvulus determine the ease and feasibility of passing a tube. Difficulties or easiness to pass a tube does not have a diagnostic value for the magnitude of the volvulus. Perforation of a compromised stomach can happen with orogastric tube. Measurement from the chin to the xiphoid before introduction of the tube may help prevent perforation of the stomach. If the intubation is successful stomach lavage is required to empty the stomach. Color and content of the fluid coming back after stomach lavage need to be looked at. Hemorrhagic fluid or black necrotic fragments are indications of stomach wall necrosis. To help intubation, it could be beneficial to place the dog in an upstanding position on its hind leg. If intubation is not successful, percutaneous gastrocentesis with 18 gauge needles is an option to decompress the stomach. Percutaneous gastrocentesis is performed on the right side. Abdominal contamination is a risk after percutaneous gastrocentesis especially if large trocars are used. After percutaneous gastrocentesis orogastric intubation can be tried again.
Cardiac rhythm needs to be evaluated before surgery. Atrial fibrillation, supraventricular tachycardia, and ventricular tachycardia are the most common arrhythmias associated with a gastric dilatation volvulus. Usually the arrhythmias occur postoperatively. Twenty-five percent of dogs presented for gastric dilatation volvulus already have cardiac arrhythmias.
Surgical intervention is required after medical stabilization of the patient. Surgical intervention is recommended as soon as possible. Delayed surgery increased the potential for gastric wall edema and necrosis, and venous stasis especially if the stomach is rotated 360o. If the surgery is delayed potential cardiac arrhythmias can develop. Seventy-five percent of the dogs that have arrhythmias develop them 36 hours after the gastric dilatation episode. If the surgery is delayed then the stomach needs to be kept decompressed by either a right sided gastrostomy or a pharyngostomy tube. If the animal is referred to a surgery center temporary decompression might be required too.
Surgical Treatment
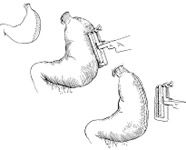
The purpose of the surgery is to derotate the stomach, evaluate the stomach wall and the spleen for ischemic injury, and perform a gastropexy. Gastropexy decreases the chances of recurrence from 80% to 5%. Dogs after a gastropexy can still dilate but not rotate their stomach.
The dogs are placed on dorsal recumbency and a midline celiotomy is performed. The surgeon stays on the right side of the dog. Upon opening the abdominal cavity, the omentum is covering the stomach which confirmed the diagnosis of gastric dilatation volvulus. The stomach can be decompressed again before derotation with a large orogastric tube. After identification of the pylorus and the fundus with a clockwise rotation, the pylorus is grasped with the right hand and pulled ventrally toward the abdominal incision while the left hand pushed the fundus dorsally into the abdominal cavity. The spleen follows the motion of the stomach. If a splenic torsion is present a splenectomy is performed without untwisting the vascular pedicle.
After derotation the stomach and spleen are evaluated for ischemic injuries. Usually the spleen shows signs of venous congestion that resorb quickly after derotation of the stomach. Splenectomy is indicated if thrombosis of the splenic artery is present. Evaluation of gastric wall perfusion is difficult. Approximately 10% of dogs have a devitalized gastric wall requiring gastrectomy. Ischemic injury occurs most commonly in the fundic area along the greater curvature. No objective criteria exist to evaluate the gastric wall. Absence of peristaltic wave, pale greenish to gray serosal color, thin gastric wall, and lack of bleeding after partial thickness incision are signs of gastric wall devitalisation. Gastrectomy is required to resect the necrotic stomach wall. Two options are available: gastrectomy with either traditional suture technique or stapling suture, or an invagination of the stomach wall.
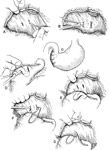
The stomach is packed off from the abdominal cavity with multiple moistened laparotomy sponges. The healthy stomach is retracted with stay sutures to prevent gastric spillage. Branches of the short gastric arteries and left gastroepiploic artery supplying the area are ligated. Necrotic gastric wall is resected with Metzenbaum scissors to the level of healthy gastric tissue. A two layers closure with inverting pattern using 3-0 absorbable monofilament suture is necessary to close the stomach. This technique is associated with a 60% mortality. If autostapling equipment is available a Gastro-Intestinal Anastomosis device (GIA 50) or a Thoraco-Abdominal device (TA 90) can be used to perform the gastrectomy (Figure 1). Advantages of this technique include decrease surgical time and decreased abdominal contamination from gastric spillage. Mortality rate with the autostapling equipment is close to 10%. Stomach rupture at the time of surgery is associated with severe peritonitis. Open abdomen lavage is recommended to treat the peritonitis. After gastrectomy, a gastropexy is required. A belt loop gastropexy is the technique of choice. It seems as efficient as the circumcostal gastropexy without the technical difficulties. (Figure 2). Two small transverse stab incisions are made in the parietal peritoneum and transverse abdominalis muscle. The incisions are located 2 to 3 cm caudal to the last rib, and one third the distance from the ventral to dorsal midline (Figure 2 A). The incisions are made 3 cm apart, and a flap of transverse abdominalis muscle is elevated (Figure 2 B) A 4 cm by 3 cm seromuscular flap is created in the pyloric antrum (Figure 2 C-D). A branch of the right gastroepiploic artery is incorporated in the flap. One simple interrupted suture can be placed between the stomach wall and the ventral part of the transverse abdominalis muscle flap (Figure 2 E). Using a stay suture, the seromuscular flap is passed through the belt loop in the abdominal wall (Figure 2 F) The seromuscular flap is sutured back on its original position using 3-0 absorbable monofilament with simple interrupted pattern (Figure 2 E).
Postoperative Complications
Several complications can occur after surgical treatment of gastric dilatation volvulus. They result from the pathophysiology of the gastric dilatation volvulus syndrome. Shock after surgery results usually from inappropriate treatment prior to surgery, surgical blood loss, anesthetic depression and fluid sequestration due to ileus. Septic shock can result from toxins and bacterial absorption from gastric mucosa necrosis and peritonitis.
Ventricular arrhythmias are common after gastric dilatation volvulus, especially 36 hours after surgery. It occurs in 50% of the cases. They result from poor myocardial perfusion, ischemia, acidosis, electrolyte imbalance, thromboembolic event, and myocardial depressant factor. Treatment requires correction of acid-base and electrolyte imbalances, and hydration. Lidocaine, 2 to 4 mg/kg as a slow bolus is given intravenously followed by a constant rate infusion of 50 to 100 microgm/kg/mn is the drug of choice for treatment of ventricular tachycardia. Procainamide intramuscularly can be used at the dose of 10 mg/kg every 6 hours.
Hypokalemia, blood loss, and disseminated intravascular coagulopathy need to be recognized and treated accordingly. Gastric rupture 3 to 5 days after surgery occurs rarely postoperatively but is due to inappropriate assessment of the viability of the stomach at time of surgery. Gastritis secondary to mucosal ischemia is treated with cimetidine or ranitidine, and sucralfate.