Monitoring anesthetized dental patients: the five-parameter approach
Clients frequently express concern about the anesthesia their dogs or cats need for professional oral evaluation and care.
Clients frequently express concern about the anesthesia their dogs or cats need for professional oral evaluation and care. Often, tooth-by-tooth assessment results in a treatment plan that requires many hours of anesthesia. Constant monitoring of the patient's physiological status is critical to a consistently positive outcome.
The American College of Veterinary Anesthesiologists (ACVA) recommends monitoring circulation to ensure the following things happen: blood flow to tissues is adequate; enough oxygen concentration is in the patient's arterial blood; and the patient's ventilation is maintained. ACVA also recommends, for legal purposes, maintaining an anesthetic record of significant events and trends in monitored parameters. Furthermore, a responsible individual, aware of the patient's status, must be available at all times during anesthesia and recovery, according to ACVA recommendations.
Monitoring is accomplished through subjective methods (e.g., clinical appearance) and objective methods (e.g., electronic systems). During anesthesia, the patient should have minimal jaw tone and no palpebral reflex. The femoral pulse should be palpable, and the perfusion time should be two seconds or less. Breathing during balanced anesthesia should be even and regular.
Anesthetic complications may be detected by electronic monitors before being recognized by a trained clinician. In these situations, seconds count. Often, the advanced warning system can head off problems before they become critical or do long-term damage. Thus, these systems provide better patient outcomes, reduce stress during the procedure and may help minimize overall procedure time.
An ideal electronic monitoring system checks for the following: blood pressure, ventilatory status, oxygenation of hemoglobin, temperature, and heart rate and rhythm (Figure 1).
Figure 1: An ideal monitoring system checks for blood pressure, ventilatory status, oxygenation of hemoglobin, temperature, and heart rate and rhythm.
Blood pressure (BP)
Ensuring the proper perfusion of the patient's vital organs is paramount in anesthetic procedures. Since perfusion is affected directly by anesthetic depth and blood volume (i.e, hydration), continuous BP monitoring to avoid hypotension is critical. A recent human study concluded that two of the three causes of death up to a year after non-cardiac surgery can be attributed to anesthetic depth and duration.1 While the gold standard for continuous BP monitoring is direct arterial pressure, it is highly invasive, which renders it impractical in clinical practice.
Three noninvasive, indirect BP methods include Doppler, plethysmography and oscillometric. Doppler does not allow for continuous, hands-free monitoring of BP trends, and it delivers a reading only on systolic pressure. Plethysmography has never been validated against direct pressure in both anesthetized dogs and cats. This leaves the oscillometric method as the best choice. Since there are veterinary-specific oscillometric BP monitors that have proven to be accurate and reliable in anesthetized dogs and cats, these are becoming the standard, just as we saw 20 years ago in human medicine.
For best results, the technician should set the oscillometric BP monitor's high and low alarms to warn of trouble, cycle the readings automatically every three to five minutes, and focus on cuff selection and placement. As a rule of thumb, the cuff diameter should be 40 percent of the circumference of the patient's limb. A cuff should be placed on the limb so it is snug and at heart level.
Normal readings for anesthetized dogs and cats are:
> systolic, 90 to 150 mm Hg;
> diastolic, 40 to 60 mm Hg; and
> mean, 60 to 90 mm Hg.
Treatment of hypotension includes decreasing the plane of anesthesia, administering fluid or increasing the rate of fluid administration, and giving an inotrope (e.g., dopamine or dobutamine by intravenous infusion to effect). If these treatments are ineffective, administration of hypertonic saline (5 mL/kg), blood or hetastarch usually will return the blood pressure to normal (Figure 2).

Figure 2: A variety of treatments may help return blood pressure to normal.
Ventilatory status
End-tidal carbon dioxide (ETCO2) monitoring through capnography often is called the "anesthesia disaster early-warning system." Vitally important, it is the only parameter that thoroughly reflects a patient's ventilatory status, and it can signal problems within two breaths. Either expired CO2 or ETCO2 will measure the CO2 produced in the cells, a function of metabolism. CO2 transported from the cells to the lungs is a function of circulation; CO2 is eliminated by the lungs.
Capnography gives a graphic and a numerical readout of the CO2 concentration in a patient's exhaled gases. It provides a means to assess ventilation, integrity of the airway, and the breathing circuit, as well as cardiopulmonary function.
A capnogram is the graphic portrayal of the changing concentration of exhaled CO2 during the respiratory cycle. P, Q, R and S are letters used to refer to different portions of the waveform. A normal waveform (Figure 3a) should have a baseline of zero during inspiration (i.e., inspiratory baseline). This is followed by an expiratory upstroke (P and Q) that contains little or no CO2 and moves the curve upward until it levels out at a plateau (Q and R). CO2 concentration continues to increase until it reaches its maximum at point R just before the onset of inhalation (i.e., inspiratory down stroke).

Figure 3a: A normal waveform has a baseline of zero during inspiration.
The height, frequency, shape, rhythm and baseline position of the waveform are monitored during anesthesia. CO2 concentration in the sample is reflected by the wave height. Changes in the standard waveform should alert the veterinarian to a problem with the patient, the airway or the anesthetic circuit. Normal readings are in the range of 35 to 45 mm Hg (Figure 3b).
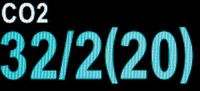
Figure 3b: Normal readings are in the range of 35 to 45 mm Hg.
Note that monitoring CO2 concentrations also allows the clinician to monitor respiratory rate. Thus, most multiparameter monitors will have a built-in apnea alarm.
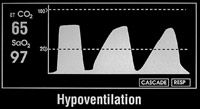
Figure 3c: Capnography results of hypoventilation in a patient.
Increased CO2 readings may be a sign of faulty check valves, an exhausted soda lime or mild to moderate patient airway obstruction, including hypoventilation (Figure 3c). Decreased CO2 readings may be a sign of hyperventilation (Figure 3d), esophageal intubation, extubation, disconnection from the breathing circuit or obstruction of the endotracheal tube.
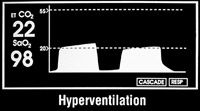
Figure 3d: Capnography results of hyperentilation in a patient.
CO2 format is a key consideration in choosing equipment. If selecting a mainstream device, ensure that the probe has no moving parts inside so it endures the rigorous environment of a busy veterinary practice. When using a sidestream device, pay close attention to the sample rate; sample rates of 50mm/min or less are recommended by the Food and Drug Administration for monitoring neonates, so this is a good goal if your practice treats small dogs and cats. If CO2 is not in your practice's budget, ensure that the monitor you purchase allows you to upgrade to CO2 later, because "plug-and-play" CO2 (Figure 4) currently is considered state of the art.

Figure 4: "Plug-and-play" CO2 currently is considered state of the art.
Oxygenation of hemoglobin
Most oxygen transported to the tissues is carried on the hemoglobin molecule. Hemoglobin travels through the blood in two forms: oxyhemoglobin and reduced hemoglobin. Pulse oximetry measures oxygen saturation via light absorption of arterial hemoglobin through pulsating blood vessels.
Oxygen saturation in an anesthetized dental patient should be maintained between 95 percent and 100 percent, particularly if the animal is breathing 100-percent oxygen. Saturation readings of 90 percent or less indicate marked desaturation, hypovolemia, shock or anemia. Possible causes include decreased oxygen flow rate, hypoventilation, diffusion impairment or shunt.
The pulse oximeter reading (Figure 5) denotes only the level of oxygen saturation and heart rate, which may be elevated when the patient hyperventilates in response to discomfort. A hyperventilating patient also may inhale excessive anesthetic gas leading to hypovolemia. Unfortunately, oximetry is an unreliable sentinel for hypovolemia, because pulse oximeters do not measure how forcefully the heart is beating. A patient with an abnormal reading may have an underlying cause that should be determined and corrected by increasing the flow rate of oxygen delivery and/or mechanically ventilating the patient until the concentration returns to normal. Moreover, pulse oximetry results are unreliable if the animal has poor perfusion, irregular heart rhythm or anemia.
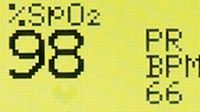
Figure 5: An anesthetized patient's oxygen saturation and heart rate.
Because excessive pigmentation and hair usually preclude accurate readings on the pulse oximeter, one of the most effective placements of the oximeter probe is on the tongue. However, dental procedures, by their nature, involve movement and instruments in the mouth, which often dislodge the tongue oximeter probe. So other areas for probe placement include the pinna, toe, prepuce, vulva, metacarpus (tarsus), digits and tail. Rectal probes also are available
Temperature
An anesthetized dental patient loses body heat to the environment because of the procedure's length, exposure to room air conditioning and use of cold irrigation solutions. Small patients are at the greatest risk, due in large part to their smaller body-surface-to-mass ratio. Some Nordic breeds (e.g., Samoyed, Siberian Husky, Alaskan Malamute) may become hyperthermic. This is why temperature control and monitoring are important for dental patients.
As the patient's temperature decreases, so does the blood pressure and heart rate. Temperature monitoring (Figure 6) can be as straightforward as a technician inserting a rectal thermometer every 10 to 15 minutes and recording results. Most multiparameter monitors allow for real-time constant digital evaluation.

Figure 6: Temperature readings for an anesthetized dental patient.
Heart Rate and Rhythm
Electrocardiography (ECG) done before and during anesthesia give the veterinarian important data regarding heart rate, rhythm and abnormal complexes. Lead2 is used primarily to monitor rate and rhythm in anesthetized patients. Continuous monitoring of the ECG pattern enables early recognition of electrical changes associated with disorders of conduction.
Electrocardiograms also can be generated using esophageal probes. While the patient is anesthetized, a probe is inserted into the esophagus until the distal electrode reaches the area dorsal to the heart base. If the ECG tracing appears small, the probe may not be inserted far enough. If inserted too deep, the tracing may appear inverted.
ECG gives minimal information on cardiac contractility and tissue perfusion. The presence of normal-appearing complexes does not indicate the patient's tissues are adequately perfused. When evaluating ECG results, first examine the rate and then the rhythm. If either is abnormal, a treatment decision usually involving the anesthesia machine settings must be made. Note that the ECG should be used with another form of monitoring (ETCO2 and/or BP) for patient evaluation during anesthesia.
In conclusion, five-parameter monitoring is a highly valuable tool in keeping patients safe during dental procedures, and it will lead to better short- and long-term outcomes, reduce the stress in the dental suite and make the veterinary operation more efficient.
Dr. Bellows owns Hometown Animal Hospital and Dental Clinic in Weston, Fla. He is a diplomate of the American Veterinary Dental College and the American Board of Veterinary Practitioners. He can be reached at (954) 349-5800; e-mail: dentalvet@aol.com.
Reference
1. Monk G, Saini V, Weldon BC, et al. Anesthetic Management and One-Year Mortality After Non-Cardiac Surgery. Anesth Analg 2005;100:4-10.