Opioids: Bolus or CRI for anesthesia (Proceedings)
Many of the anesthetic agents commonly used today, including thiopental, propofol, isoflurane, and sevoflurane, have little intrinsic analgesic activity.
Objectives of the presentation:
To describe the onset, duration, and pharmacologic effects of opioids in dogs and cats.
To discuss expected effects of opioids in combinations for sedation before anesthesia.
To illustrate options for use in bolus and continuous infusion administration using case examples.
To emphasize potential adverse effects and precautions for satisfactory anesthetic outcomes.
Summary of important points:
Balanced anesthesia utilizes several agents to produce the best quality anesthesia and uses smaller drug doses to create a wider safety margin.
Opioids exert their effect through one or more receptor types, therefore, interactions between 2 opioids administered concurrently can produce undesirable effects.
Wide variation in response to butorphanol and buprenorphine observed in cats.
Rapid IV injection during anesthesia can decrease blood pressure
Respiratory depression is the major adverse effect of opioid administration
Hypoxemia can develop when dogs of cats are heavily sedated and breathing air
Reason to use opioids
Many of the anesthetic agents commonly used today, including thiopental, propofol, isoflurane, and sevoflurane, have little intrinsic analgesic activity. Consequently, large doses of these agents must be administered in order to achieve a patient that is non-responsive to surgical stimuli. Unfortunately, these agents have a depressive effect on the cardiovascular system with increasing depth of anesthesia. Balanced anesthesia is created when small doses of several drugs are administered to achieve unconsciousness, relaxation, analgesia, and lack of response to stimuli, a situation that provides safer as well as satisfactory anesthesia for our patients.
Lack of analgesia during a surgical procedure induces long-lasting changes in the neurons in the spinal cord and increased pain awareness post operatively. Hyperalgesia is when the central nervous system is sensitized resulting in easier activation of the pain pathways. Hyperesthesia occurs when pain pathways can be activated by normally non-noxious stimuli. Analgesic agents should be administered pre-emptively to modify these adverse changes, that is, before the start of surgery and continued for at least 8 hours after the end of anesthesia. Additional analgesia, and sometimes sedation, may be required for longer depending on the nature of the surgery and the temperament of the animal.
It is generally accepted that no single analgesic agent provides effective pain control for all types of pain. Frequently, 2-5 analgesic agents or techniques (multimodal analgesia) are administered to a single patient in an attempt to manage surgical pain. Agents employed include non-steroidal anti-inflammatory agents, systemic administration of opioids, nerve blocks with local anesthetic solutions and/or opioids, and continuous intravenous infusions of lidocaine or ketamine.
Opioids exert their effects through receptors on primary sensory neurons in the periphery and in the central nervous system. The opioids may interact with one or more receptors to produce a variety of effects. Agents that are agonists at the mu (µ) receptor include morphine, fentanyl, hydromorphone, oxymorphone, and methadone. mu receptors mediate analgesia, sedation, euphoria, dependence, miosis, respiratory depression, bradycardia, gastrointestinal effects such as vomition and ileus, biliary duct constriction, urinary retention and pruritis. Buprenorphine is a partial mu agonist because it has high affinity for mu receptors but its activity is less than morphine. It has been reported that buprenorphine is a partial antagonist of kappa receptors. Meperidine is a mu receptor agonist with some activity at delta and 5-HT receptors. Butorphanol, pentazocine, and nalbuphine are mixed agonists and antagonists. They are kappa (?) receptor agonists and partial mu agonists with moderate affinity without activity and, therefore, act as mu antagonists. Kappa receptors mediate analgesia, dysphoria, miosis, and antitussive effects. Naloxone is an opioid antagonist at mu, kappa and delta receptors.
It can be seen from these varied receptor effects that mixing of opioids in the same patient must be done carefully to avoid antagonism and lack of analgesia. A mu agonist opioid, such as morphine, oxymorphone, hydromorphone and fentanyl, should not be administered concurrently with butorphanol or buprenorphine. In a study of dogs anesthetized for ovariohysterectomy, premedication with buprenorphine blocked the analgesic effect of a mu receptor agonist given to maintain the dogs unresponsive to surgical stimulation until 2.5 times as much opioid was administered compared with patients that had not received buprenorphine. The simultaneous administration of butorphanol and buprenorphine is not advisable. Experimental studies in cats have revealed that simultaneous administration of hydromorphone and butorphanol, or butorphanol and buprenorphine resulted in little or no analgesia in some cats for 2 hours after administration. In another study, injection of butorphanol, 0.4 mg/kg, to dogs 25 minutes after administration of oxymorphone was almost as effective antagonist as naloxone.
The time of onset of action varies with the agent, the route of administration, and the dose rate. Intravenous (IV) administration results in a more intense effect for a shorter time than does intramuscular (IM) injection. The duration of each agent may be different between dogs and cats, and may be prolonged when given in conjunction with inhalation anesthesia which slows elimination of the drug. When multiple doses or continuous infusion have been given to a patient, slow release from tissues can result in persistent sedation. Onset of action and duration of commonly used opioids in healthy dogs given below.
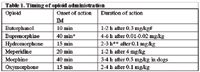
Table 1. Timing of opioid administration
#Large variation in response to butorphanol documented in cats; experimental studies have recorded elevated threshold to experimental pain for several hours in some cats.
*Onset time IM buprenorphine in published literature is 35-60 min and for IV administration is 30 min.
**Effect of hydromorphone in cats appears to be longer than in dogs, with a 0.1 mg/kg IM dose producing 5-6 hours of analgesia. Duration after IV administration is shorter: IV hydromorphone 0.05 mg/kg produced 80 min of analgesia, IV dose of 0.1 mg/kg produced significant effects for 2 h 40 min.
Opioids administered alone for premedication to general anesthesia may or may not provide sedation but will decrease the dose rate of injectable anesthetic agents for induction of anesthesia, for example, buprenorphine premedication decreased the dose rate of propofol from an average of 5.0 mg/kg to 3.3 mg/kg in healthy dogs, and butorphanol decreased the isoflurane % required for anesthesia by 19% for 60 min in healthy cats. An opioid is frequently used in combination with a sedative (diazepam or medetomidine) or tranquilizer to improve the quality of sedation. In some combinations the sedative is added to counter an effect of the opioid, such as CNS stimulatory effects in cats. In some combinations the opioid is added to counter an adverse effect of the sedative, such as unpredictable arousal of dogs sedated with medetomidine (Domitor). A beneficial effect of prior administration of acepromazine by 20 min is that it significantly decreases the incidence of vomiting after morphine and hydromorphone.
The degree of sedation obtained from a combination of sedative or tranquilizer and an opioid (neuroleptanalgesia) depends on the agents, route of administration, dose rate of agents, and the patients' physical status. Hydromorphone and oxymorphone are comparable in their effects on the cardiovascular system. Both these agents provide more analgesia than butorphanol. Acepromazine and butorphanol or buprenorphine usually induce mild sedation but in a healthy large breed dog acepromazine, 0.03 mg/kg IM, with morphine, 0.5 mg/kg IM, can induce heavy sedation. Medetomidine sedation is more easily controlled by dose rate. For example in a healthy dog, medetomidine, 10-20 micrograms/kg IM, with butorphanol, 0.2 mg/kg IM, will produce mild to moderate sedation and medetomidine, 40 micrograms/kg IM, with butorphanol produces heavy sedation, sometimes to the extent of allowing endotracheal intubation. Medetomidine has such a profound effect on the cardiovascular system that a reduced rate (50%) should be used in senior patients. Combinations of medetomidine, 20 micrograms/kg, with hydromorphone, 0.1 mg/kg, or butorphanol, 0.2 mg/kg, or buprenorphine, 0.01 mg/kg, IM in dogs produce cardiovascular effects similar to those induced by medetomidine alone, but respiratory depression is greater. The addition of midazolam, 0.25-0.5 mg/kg IM, produces an obvious increase in depth of sedation.
Severe sedation and sedation/analgesia sufficient for minor surgical procedures can be achieved by IM administration of an opioid such as butorphanol, 0.2-0.3 mg/kg, or buprenorphine, 0.01 mg/kg, or hydromorphone, 0.05-0.1 mg/kg, or (in dogs) morphine, 0.2-0.5 mg/kg, with medetomidine, 20-40 micrograms/kg, and either ketamine, 4.4 mg/kg, or Telazol, 4 mg/kg. In cats, the medetomidine dose may have to be increased to 60 micrograms/kg for total intravenous anesthesia. Onset of action of these drug combinations is quick and the animals should be kept under observation for excessive respiratory depression. The animals should be in an obstacle-free environment as unnatural body positions in conjunction with a litter tray, blanket, or water bowl can result in airway obstruction or aspiration of foreign material. Subsequent administration of inhalant anesthetic should be very low.
Simple combinations for preanesthetic sedation prior to the use of thiopental, propofol, or diazepam-ketamine for induction of anesthesia in healthy animals are acepromazine, 0.02-0.05 mg/kg, and an opioid IM (at previously stated dose rates). Midazolam IM or IV or diazepam IV can be substituted for the acepromazine. The degree of sedation achieved will depend on the patient, and the dose of subsequently administered anesthetic agents should be adjusted accordingly. Some opioids have a short duration of action and supplemental doses can be given during anesthesia, usually at half the dose rate used for premedication. When given IV during anesthesia, the opioid dose should be given in increments over several minutes to avoid acute cardiovascular depression. Choice of opioid for postoperative use must consider the duration of opioid effect and the need for additional medication. Signs associated with pain are present in animals without pain medication for at least 8 hours after surgery. Butorphanol, meperidine, and hydromorphone are too short acting for one administration to be sufficient. Preoperative administration of a NSAID such as carprofen is insufficient alone to abolish pain response to surgery.
Opioids can also be administered by continuous infusion. This insures a constant blood level of drug, a smooth plane of anesthesia, and a reduction in percent of isoflurane or sevoflurane. Morphine and fentanyl are the two most commonly used opioids for this purpose. The drugs can be added to a 500 ml bag of fluids or administered from a syringe that is controlled by a syringe pump. Fentanyl can be administered as a bolus of 2 µg/kg at 20 minute intervals or infused at 6 µg/kg/hour. Fentanyl (Sublimaze) is available as 50 µg/ml and the infusion rate using a syringe pump for a 20 lb dog would be 1 ml/hour. Microinfusion extension sets (Medex product No. 536040), 60 inches long with small 0.3 ml internal volume, are useful for syringe pump administration of fentanyl. Heart rate may progressively decrease, which sometimes should be treated by administration of glycopyrrolate, and artificial ventilation may be necessary to treat respiratory depression. The infusion rate is usually reduced by half for recovery and can be continued for up to 12 hours post-operatively. Some sedation may be included when appropriate, for example, IV acepromazine, 0.01-0.02 mg/kg, or medetomidine, 2-3 µg/kg, or an infusion of diazepam, 0.5 mg/kg/h.
Morphine can be administered to dogs as a continuous infusion of 0.2 mg/kg/hour. Using a syringe pump this would be 0.12 ml/hour/10kg (22 lb) (15 mg/ml Morphine). Administration is easier if the total dose for 2-3 hours (or anticipated surgery time) is drawn into a syringe and diluted to provide a dose rate of 5 ml /hour. Alternatively, 10 mg morphine can be added to a 500 ml bag of lactated Ringer's solution (0.02 mg morphine /ml) and dripped to give 10 ml/kg/h. This rate will provide anesthesia fluids and morphine at 0.2 mg/kg/h. It must be remembered that this bag cannot be used to give a bolus of fluid for cardiovascular support. A study of experimental dogs anesthetized with isoflurane determined that morphine CRI at 0.2 mg/kg/h decreased the requirement for isoflurane by 48%. Steady state plasma concentrations were reached by 2 hours of infusion when a loading dose was not administered (however, premedication with morphine or hydromorphone will substitute for a loading dose). The same study found the addition of lidocaine and ketamine to the morphine resulted in no further decrease in isoflurane concentration. Lidocaine alone (0.05 mg/kg/h) decreased isoflurane requirement by 29%. Note that in this study all dogs were artificially ventilated. It is advisable to decrease the infusion rate by half for the last 30 min of anesthesia and the dogs must be observed closely for respiratory depression and hypoxemia (pulse oximeter) when taken off oxygen at the end of anesthesia. An alternative stronger formula for MLK is 500 ml LRS + 1.6 ml (15 mg/ml) morphine + 15 ml 2% lidocaine + 0.6 ml ketamine, infused at a rate of 5 ml/kg/h or 2.2 ml/lb/h (note the different rate) provides morphine 0.24 mg/kg/h, lidocaine 0.05 mg/kg/min, and ketamine 0.6 mg/kg/h.
A fentanyl transdermal patch (Duragesic or Mylan) will provide a constant blood level without injections. Fentanyl is absorbed through the skin to produce a full onset of effect by 24 hours after application in dogs and a maintained blood level of 72 hours. The patch should be applied the day before surgery for maximum utility. An experimental evaluation of a 25 µg/hour patch applied to cats 24 hours before anesthesia discovered that isoflurane requirement was decreased by an average of 18%. Sustained plasma fentanyl concentrations were achieved in these cats by 12 hours after patch application. Other studies have measured sustained plasma concentrations in cats for 100 hours.
Adverse effects and precautions
Some opioids, principally morphine, meperidine, hydromorphone, and oxymorphone consistently induce vomiting when given to healthy dogs. While this may be unpleasant for the dogs, it may not be a significant problem except in patients with gastrointestinal obstruction, cervical pain, laryngeal paralysis, upper airway obstruction, deep corneal ulcers, and increased intracranial pressure. Gastroesophageal (GER) reflux in anesthetized patients is of concern because of the potential for esophageal stricture formation later. In a study of clinical patients scheduled for orthopedic procedures, the incidence of vomiting was 50% in dogs given acepromazine and morphine, 0.22 mg/kg, and 60% in dogs given ace and morphine, 1.1 mg/kg. GER was present in 26% of acepromazine-only dogs and 60% in the group of high dose morphine dogs. GER was first recorded about 10 min after induction of anesthesia and was not related to whether the dogs had vomited from their premedication.
Opioids can cause significant respiratory depression, especially when used as part of a general anesthetic protocol. Opioids decrease the central nervous system response to carbon dioxide and to hypoxia, and depress the center responsible for respiratory rhythm. Breathing can become periodic with long pauses. Respiratory rate may be within the normal range but frequently the depth of breathing is shallow, resulting in hypercarbia. Oxygenation is rarely a problem when the patient is intubated and connected to an anesthesia machine breathing oxygen. In patients with opioid-induced respiratory depression, hypoxia may develop within 20 minutes after the animal is disconnected from the machine and allowed to breathe room air.
A major advantage of opioids in general compared with other anesthetic agents is that they cause minimal depression of left ventricular contractility and do not sensitize the myocardium to catecholamines. However, a decrease in central sympathetic outflow may cause vasodilation and decrease in blood pressure. The venodilator effect typically develops later than arteriolar vasodilation and lasts longer. Hypotension may develop after rapid IV injection of morphine or meperidine due to histamine release and direct vasodilatory effect on blood vessels. Any opioid, including butorphanol and buprenorphine, may cause an abrupt decrease in blood pressure when injected IV into a patient with an unstable cardiovascular system, such as after considerable hemorrhage or in senior patients.
Some opioids, fentanyl, hydromorphone, and oxymorphone in particular, may cause bradycardia and concurrent administration of glycopyrrolate, 0.01 mg/kg IM or 0.005 mg/kg IV, may be advisable. Heart rates are less likely to decrease below 60 beats/min during anesthesia and post-operative vomiting is less when an anticholinergic is used. Bradycardia may develop as the drug effects fade, after 1.5 hours with atropine and 2-3 hours with glycopyrrolate, or when the animal gets cold.
Heart rates are less elevated after glycopyrrolate than after atropine and, therefore, glycopyrrolate may be the better choice in senior animals, and in animals with mitral insufficiency or mild cardiac disease. Specific contraindications to use of anticholinergic drugs are animals with severe cardiac disease that cannot tolerate tachycardia, such as cardiomyopathy or ventricular dysrhythmias, with preexisting tachycardia, and with a fever greater than 103.5o (atropine crosses the Blood-Brain Barrier and interferes with thermoregulation).
Hyperthermia has been a reported complication of the use of hydromorphone in cats and the effect may last for up to 5 hours. The cats' temperatures rapidly decrease after administration of naloxone.
Podcast CE: A Surgeon’s Perspective on Current Trends for the Management of Osteoarthritis, Part 1
May 17th 2024David L. Dycus, DVM, MS, CCRP, DACVS joins Adam Christman, DVM, MBA, to discuss a proactive approach to the diagnosis of osteoarthritis and the best tools for general practice.
Listen