Surgery STAT: Use of adult stem cells in clinical orthopedics
Adult stem cells are cells found in various tissues throughout the bodies of both juveniles and adults. By definition, these are undifferentiated cells that can be induced to become any of a number of cell types.
Adult stem cells are cells found in various tissues throughout the bodies of both juveniles and adults. By definition, these are undifferentiated cells that can be induced to become any of a number of cell types.
Bone marrow-derived mesenchymal stem cells (BMDMSC) are of particular interest in orthopedics, as cells in this lineage are involved in osteogenic differentiation of cells at the site of bone generation and regeneration. These cells, by virtue of their presence in bone marrow, have easy access to sites of fracture healing. However, only a small fraction (fewer than 0.01 percent) of the cells present in bone marrow are BMDMSC. A bone-marrow aspirate may contain only a few thousand cells — not nearly enough to incite a significant osteogenic effect. For this reason, investigators are expanding these cells in culture to obtain numbers in the millions. This number of cells has been shown to induce bone healing.
Bone marrow-derived mesenchymal stem cells can be obtained by harvesting bone marrow from a patient and growing that patient's cells in culture in a laboratory. These are referred to as autogenous cells, or cell autografts. However, this is difficult to do in a clinical setting in which a laboratory is not available for immediate transfer of the marrow sample. There is a large body of experimental data demonstrating the safety and efficacy of BMDMSC from another individual of the same species, referred to as cell allografts. This newer approach will make it easier for clinicians in various practice settings to use these cells, not only for bone healing, but for regeneration of other tissues as well.
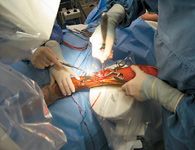
Photo 1: Dèbridement of fibrous tissue from a delayed union site using a bone curette through a limited approach.
Bone marrow-derived mesenchymal stem cells can be grown in a commercial or academic laboratory and used by veterinarians at distant locations by shipping the cells via overnight courier on dry ice.
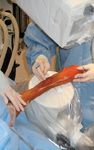
Photo 2: Identification of site of dèbridement and stem cell delivery using a percutaneous approach with fluoroscopic guidance.
There are a number of clinical scenarios in which a minimally invasive, biologic approach to tissue regeneration carrying low risks is desirable. These cells can be delivered to virtually any anatomic site via a percutaneous approach. For example, this system can be utilized in any delayed or non-union fracture site, as well as in slowly fusing arthrodesis sites.
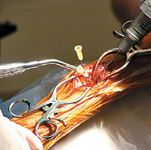
Photo 3: Providing access to marrow elements at the site of impaired bone healing by drilling small holes in sclerotic bone.
The delivery site should first be dèbrided (Photo 1) to remove all fibrous tissue that may impede osteogenesis. This dèbridement can be accomplished with a percutaneous approach using fluoroscopic guidance (Photo 2), or a limited open approach. Provide access to the marrow cavity by drilling small holes in any sclerotic bone at the site (using 0.045-inch Kirschner wires, Photo 3). This preparation provides access to the patient's own stem cells, which may undergo osteogenic differentiation in response to delivery of the allograft BMDMSC. The allograft BMDMSC can be delivered through the same percutaneous or limited open approach (Photo 4), further compromise the vascular supply to the bone and soft tissues.
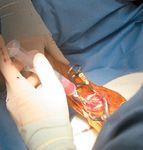
Photo 4: Delivery of stem cells via a limited approach
The cells (Photo 5) can be delivered in a number of carrier systems designed to retain the cells at the site of interest, to make delivery through a percutaneous approach easy and safe and to augment the osteogenic potential of the cells.
Photo 5: Allograft stem cells delivered from an academic or commercial laboratory following expansion in vitro.
Examples of such carrier systems that have been used with success with BMDMSC include: absorbable collagen sponge, absorbable gelatin sponge or powder, autograft cancellous bone, allograft cancellous bone, autograft and/or allograft corticocancellous bone, autologous bone marrow and any combination of these carriers (Photo 6). Radiographs should be taken four weeks status post stem-cell delivery to assess the progress of bone healing. The need for further follow-up will vary depending on the nature of the case, the age of the patient and the robustness of the initial response to stem-cell therapy.
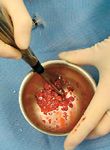
Photo 6: An example of a mixed carrier system for delivery of stem cells.
Dr. Zachos is an ACVS board-certified surgeon. She is assistant professor of orthopedic surgery at Michigan State University, where she is an attending orthopedic surgeon in the Small Animal Clinic of the Veterinary Teaching Hospital. She also runs a molecular orthopedics laboratory at Michigan State. Her research focus is the development of translational cell-based gene therapy techniques for bone and cartilage regeneration.
Her work has been recognized by the American Academy of Orthopaedic Surgeons, the Orthopaedic Research Society, the Ruth Jackson Orthopaedic Society and the 7th International Conference on Bone Morphogenetic Proteins.
Dr. Smith is a resident in small-animal surgery at the Lois Bates Acheson Teaching Hospital, Department of Clinical Sciences, College of Veterinary Medicine, Oregon State University.