News
All series
Advertisement
Advertisement
Trending on dvm360
1
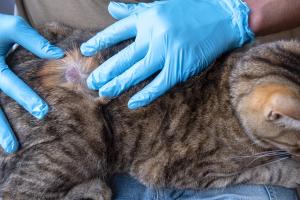
New guidelines for feline oral health and dental care are released
2
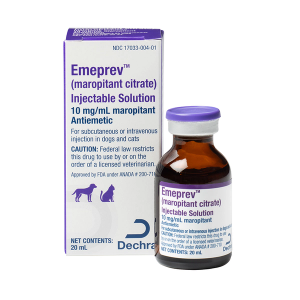
FDA approves maropitant citrate injectable solution
3
Key takeaways: Practical approaches to canine lymphoma diagnosis and management
4
Sneak peek: FDA approves its first bioequivalent injectable solution used for antiemetic feline and canine patients, and other news
5