Acute respiratory distress-what to do when they are so blue (Proceedings)
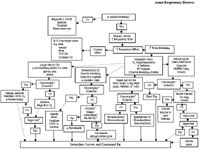
Acute respiratory distress (ARD) is the sudden onset of rapid and/or labored respiratory. It can be caused by pathology or obstruction associated with the nasal passages, oral cavity, pharynx, larynx, trachea, bronchi, alveoli, pulmonary vasculature or lymphatics, pulmonary innervation, chest wall, diaphragm, or pleural space.
Definition/etiology
Acute respiratory distress (ARD) is the sudden onset of rapid and/or labored respiratory. It can be caused by pathology or obstruction associated with the nasal passages, oral cavity, pharynx, larynx, trachea, bronchi, alveoli, pulmonary vasculature or lymphatics, pulmonary innervation, chest wall, diaphragm, or pleural space.
Pathophysiology required
Respiratory distress occurs in the following order of progression from least to most severe: increased respiratory rate; change in breathing pattern with increased work of breathing; change in posture; open mouth breathing; cyanosis (paO2 < 60 mmHg); death. Cyanosis, severe work of breathing (active intercostal muscle contraction for movement of the rib cage, open mouth breathing with lips retracted at the commissures), barrel chested appearance of the rib cage, and/or blood or foam coming from the trachea are signs of catastrophic acute respiratory distress. These animals are dying before your eyes. Severe work of breathing is manifested by using the abdominal muscles and diaphragm for chest movement. The amount of work required will be dependent upon how much of the lung or thorax is involved and whether the problem is acute (more work required) or chronic.
Key points primary survey
- Determine severity of ARD – catastrophic, severe or mild
- Breathing patterns suggests anatomical location of pathology
- Assess perfusion and hydration.
Primary survey
Immediately determine if the animal is breathing. If not, intubate and ventilate. Determine the degree of respiratory distress (catastrophic, severe, mild). In an acute severe or catastrophic situation, the animal will assume a body position of relief.
The dog will want to stand with elbows abducted and back arched. As the pathology progresses, the dog will extend his neck and open mouth breath. The cat will tuck the front and rear legs and feet tightly under their body and arch their backs elevating their sternum off of the surface. With chronic distress the cat does not have a specific position of relief
It is important to determine the location of the pathology.(large airway, pleural space disease, parenchymal disease, small airway) by observing the pattern of breathing and careful auscultation. Observation of the rib cage-abdomen junction is ideal to determine whether the chest and abdomen are moving together, in the same direction, or if they are moving in opposition. It is also important to note inspiratory to expiratory time ratio (normally 1:2).
If breathing is loud, heard without the aid of a stethescope, large airway obstruction is the likely cause. Castrophic large airway obstruction presents with severe cyanosis and cardiovascular compromise. The breathing sounds may not be loud if the animal is losing consciousness or is exhausted from trying to breath against an obstruction. Inspiratory stridor suggests upper airway pathology and expiratory stridor suggests lower airway pathology. Stridor on both inspiration and expiration suggests either involvement of the entire trachea such as tracheal collapse or a fixed obstruction such as a mass.
Rapid, shallow breathing with the chest and abdomen moving together, in the same direction at the same time is most compatible with lung parenchymal disease. Auscultation will find louder than normal lung sounds with early disease and crackles and rales in the area of involvement with severe disease. Cats will have louder than normal lung sounds as their primary auscultatory finding unless the lung involvement is severe.
An irregular breathing pattern, with the chest and abdomen moving in opposition to one another is most compatible with pleural space disease. Auscultation may find dull or muffled lung sounds in the location of the pathology. Percussion in large breed dogs can find an area of dullness at the site of the pleural space pathology. Lung sounds can be normal in a cat with pleural space disease. Catastrophic tension pneumothorax will present with the animal having a barrel chested appearance and severe cardiovascular compromise. There will be little chest wall movement. A noncompressible anterior mediastinum suggests an anterior mediastinal mass in the cat.
Short inspiration and prolonged expiration, with an expiratory push of the diaphragm, is compatible with small airway disease. Auscultation should find high pitched wheezes.
Rapid breathing with normal effort will suggest non-respiratory tract related causes such as pain, CNS disease, peripheral nerve pathology, neuromuscular disease, muscle pathology, or metabolic acidosis.
A brief history is immediately obtained to determine if there is known trauma, exposure to rodenticides, past medical history of heart disease or recurrent breathing problems, signs of illness other than ARD, or recent vomiting.
Key points resuscitation
- Minimize stress
- Provide flow-by oxygen immediately
- Place intravenous catheter
- Provide anesthesia or sedation
- Resuscitate prior to diagnostics if catastrophic or severe ARD
- Further procedures specific to location of pathology
Resuscitation
Resuscitative intervention is required most often prior to aggressive diagnostics to minimize stress. All animals with ARD will receive: oxygen supplementation by flow-by, mask or bag oxygen administered during resuscitative procedures and an intravenous catheter. Immediate and aggessive resuscitative intervention is essential for animals presenting with catastrophic ARD.
Tension pneumothorax with severe cardiovascular compromise will require an immediate small incision into the pleural space, converting to an open pneumothorax and relieving the tension. Rapid induction anesthesia or neuromuscular blockade with anxiolytic agent, intubation, and positive pressure ventilation are initiated.
Large airway obstruction requires rapid anesthetic induction or neuromuscular blockade with anxiolytic agent, rapid intubation and ventilation with 100% oxygen. If there is a total upper airway obstruction, an emergency tracheotomy is performed below the obstruction. Transtracheal oxygen can be administered while preparing for the tracheotomy.
Parenchymal disease with bloody foam coming from the trachea will require rapid anesthetic (etomidate induction or neuromuscular blockade with anxiolytic agent, intubation, positive pressure ventilation with 100% O2 and airway suctioning.
Small airway disease in the cat with status asthmaticus will require intravenous glucocorticosteroids, parenteral bronchodilators such as terbutaline or aminophylline (if no evidence of hypertrophic cardiomyopathy), and nebulization with bronchodilators. Epinephrine at 0.25 ml/cat of a 1:10,000 dilution can be given IM or slowly IV for rapid bronchodilation.
Most animals with ARD will require sedation (butorphanol 0.2 – 0.8 mg/kg IV) if the animal is anxious and distressed unless the animal will receive rapid induction anesthesia and intubation. Initial resuscitative procedures with mild to severe ARD will depend upon severity and initial localization of the pathology based upon respiratory pattern and auscultation. Large airway disease requires a patent airway. Sedation and supplemental oxygen is administered at high flow rates until further diagnostics are completed (see stridor and airway obstruction).
Parenchymal disease requires: careful auscultation for a heart murmur or gallop or persistent arrhythmia suggestive of cardiogenic edema. If heart failure is suspected, administration of furosemide (7 mg/kg IV) and nitroglycerine ointment on bare skin over the chest is administered and the animal allowed to stabilize prior to further diagnostics. Animals with parenchymal disease without evidence of heart disease or pneumonia can benefit from a one time administration of furosemide (2-7 mg/kg IV) until further diagnostics. Fever, history of vomiting, and/or localized lung involvement on auscultationsuggests possible pneumonia and diuretic administration is delayed until further diagnostics unless the distress is severe.
Pleural space disease requires thoracentesis done dorsal to the costochondral junction if air is suspected or ventral to this level if fluid is expected. Often ultrasound can help guide thoracentesis if the fluid is loculated and difficult to aspirate, saving any fluid recovered for culture/sensitivity and cytology. A diaphragmatic hernia is to be suspected when there is a history of trauma in an animal with pleural space breathing pattern and fluid or air can not be recovered from thoracentesis.
Small airway disease is most prevelent in the cat, but can complicate parenchymal disease when there is bronchogenic pneumonia or cardiogenic pulmomary edema in either the dog or cat. Bronchodilators can be given parenterally or terbutaline can be given by nebulization. In the cat, glucocorticosteroids are given when asthma is suspected.
Secondary survey
A complete history is obtained to further defined previously mentioned problems or to discover new possible causes for the ARD. The presence of exercise intolerance or a soft cough can suggest a cardiac etiology. Harsh coughing, sneezing or nasal discharge can suggest an infectious etiology. An illness similar in other pets in the household or in relatives of this pet can direct diagnostic endeavors looking for similar pathology.. Vaccination and parasite control history (heartworm and intestinal parasites) reveal likelihood of those etiologies. Exposure to stray or unfamiliar animals may direct diagnostics towards a contagious infectious disease such as distemper, feline viral URI, etc.
A complete physical examination will now concentrate on systemic effects of the ARD and possible underlying abnormalities. Careful auscultation of the heart for murmurs, gallops or arrhythmias is done with evaluation of the perfusion status through heart rate, capillary refill time, pulse quality, mucous membrane color. The perfusion status will can reflect the impact of the respiratory pathology on the body. Careful thoracic ausculation finds subtle changes post resuscitation, looking for muffled heart or lung sounds (suggestive of pleural space disease), louder than normal lung sounds, crackles or rales (suggestive of parenchymal disease), high pitches wheezes (suggestive of small airway disease), or areas of dull lung sounds (suggesting a space occupying mass or collapse or consolidation of lung in the area of dullness).
Other physical examination procedures include: palpation of the trachea for any irregularities or stimulation of coughing suggesting tracheal irritation; palaption of the skin for any evidence of subcutaneous emphysema suggestive of chest wall, thoracic inlet or airway rupture; palpation of the chest wall for deviations, wounds, or painful regions suggestive of rib fracture or pathology; palpation of the abdomen to estimate the presence of the abdominal organs (often organs missing with diagphragmatic hernia) and for evidence of any masses or organ abnormalities which might contribute to the respiratory pathology (tumor metastasis, causes of vomiting and aspiration, etc.); careful examination of the animal for signs of trauma; and rectal temperature can reflect possible inflammation or infection if elevated or poor perfusion if low.
Differential diagnosis
Causes of large airway disease include: obstruction by foreign body, mass or anatomical defect; airway rupture; collapsing trachea, laryngeal paralysis, smoke inhalation; infectious diseases; Causes of parenchymal disease include: cardiogenic pulmonary edema; non-cardiogenic pulmonary edema (to include intravascular volume overload); smoke inhalation; pneumonia; pulmonary thromboembolism; acute respiratory distress syndrome; pulmonary contusions or hemorrhage; lung atelectasis; pulmonary neoplasia; pulmonary granulomatous disease; pulmonary infiltrates with eosinophils.
Causes of pleural space disease include: pneumothorax; hemothorax; pyothorax; chylothorax; pleural space or mediastinal masses; pneumomediastinum; diaphragmatic hernia; right heart failure; obstruction of vena cava; neoplasia; heartworm disease; hypoalbuminemia. Small airway disease include feline asthma; bronchiectasis; emphysema; chronic obstructive lung disease; bronchogenic pneumonia; cardiac failure.
Chest wall or muscles; rib fractures; flail chest; diaphragmatic hernia; electrolyte imbalances (hypokalemia; hyperkalemia; hypocalcemia; hypomagnesemia); pathology of phrenic nerve; brain stem or cervial or thoracic spinal cord disease; peripheral neuropathy; neuromuscular disease; muscle disease. Nonrespiratory causes include systemic disease, pain, metabolic acidosis. Causes of respiratory distress that develops in a hospitalized patient include: intravascular volume overload, aspiration pneumonia, onset of heart failure or arrhythmias, pulmonary thromboembolism, hematogenous pneumonia, hemorrhage, atelectasis, and acute respiratory distress syndrome.
Diagnostic tests
1. Provide oxygen supplementation during diagnostic procedures.
2. Have equipment prepared in advance before bringing the animal into the area for the diganostic procedures.
3. Thoracentesis if performed in animals suspected to have pleural space disease.
a. Quantities of air or fluid removed should be recorded. Later taps will indicate recurrence rates and suggest need for chest tube.
b. Recovered fluid should be submitted for fluid analysis, cytologic examination and for culture and sensitivity.
c. Ultrasound may aid in the recovery of loculated pleural fluid.
4. Radiographs of chest and other affected body regions looking for abnormalities to include: pleural air or fluid; pleural fissure lines; masses; pulmonary infiltrates; alveolar lung pattern; bronchiolar markings; rib structure; diaphragm structure; airway structure; mediastinal structures; diameter of the vena cava; heart size and shape; evidence of heartworm pathology;
a. Do not radiograph an animal in catastrophic or severe distress prior to stabilization.
b. Allow the animal to assume the position that causes the least amount of distress - a DV view of the chest might be all that is possible.
c. Neck films can be required if there is upper airway pathology.
d. Abdominal films are indicated if concerned about diaphragmatic hernia, neoplasia, granulomatous lung disease; ARDS; systemic disease;
e. Fluoroscopy can aid with lung aspirates or detecting tracheal collapse.
5. Ultrasound evaluation can be done for pleural fluid, pericardial fluid, heart disease, mass lesions, and fluid centesis.
6. Laboratory tesing can include:
a. Immediate EDB, ACT and platelet estimate, arterial blood gas analysis
b. CBC, biochemical profile, fluid analysis, urinalysis, coagulation profile
c. Aspiration of mass or affected lung and cytologic examination of fluid.
a. Ultrasound or fluoroscopy can aid in sample collection.
d. b. The animals must be watched carefully for evidence of hemorrhage or pneumothorax post procedure.
e. Lung aspirate is indicated when the pathology does not involve the small airways or is very focal in the lung tissue.
7. Transtracheal wash is indicated once the animal is stable to assess the cells and organisms in the small airways when there is lung or airway disease that produces fluid or exudate.
8. Bronchoscopy exam is indicated if TTW has been unproductive, if the lung disease is not producing fluid or exudate, or if the pathology appears to involve only focal lung lobes or airways.
Continued treatment
More efficient means of oxygen delivery such as nasal cannula or prongs is considered and fluid therapy plans are reassessed to ensure adequate perfusion and hydration. Use measures necessary to prevent vomiting and aspiration that can occur secondary to aerophagia. Aspirate gastric air if aerophagia is significant. A nutritional support plan is initiated if prolonged illness anticipated. Treat concurrent problems to include hypoalbuminemial, sepsis, coagulation defects, etc.
Monitoring and nursing care specifics
Minimize stress at all times and have all the equipment and medications necessary for diagnosis and treatment prepared in advance before performing the task to shorten procedural time and minimize stress. If the animal is presented in catastrophic or severe condition, or if the animal is progressing, have appropriate sized endotracheal tubes and laryngeoscope by the cage as well as any equipment necessary depending upon etiology (e.g. thoracentesis apparatus; suction catheter and equipment). Assure that animal is getting prescribed amount of oxygen in their cage and during diagnostic or therapeutic procedures.
Common patient monitoring includes; respiratory rate, pattern and effort, auscultation of lungs and heart, perfusion parameters, chest tube production of air or fluid. Observe posture and anxiety level of animal each time you pass the cage Equipment monitored parameters include: pulse oximetry, end-tidal CO2, ECG, blood gases, blood pressure, blood parameters to include PCV,TS, electrolytes, air oxygen concentration if using cage oxygen
Podcast CE: A Surgeon’s Perspective on Current Trends for the Management of Osteoarthritis, Part 1
May 17th 2024David L. Dycus, DVM, MS, CCRP, DACVS joins Adam Christman, DVM, MBA, to discuss a proactive approach to the diagnosis of osteoarthritis and the best tools for general practice.
Listen











