Advances help in treatment of canine GME; prognosis poor
Granulomatous meningoencephalomyelitis (GME) is a relatively common nonsuppurative inflammation of the brain, spinal cord, and meninges. The etiology is unknown.
Granulomatous meningoencephalomyelitis (GME) is a relatively common nonsuppurative inflammation of the brain, spinal cord, and meninges. The etiology is unknown.
Neurological signs of GME may be multifocal or focal, according to the localization of the lesion(s) within the CNS.
Behavior abnormalities, seizures, compulsive pacing, circling, or cranial nerve abnormalities may be observed. Choroiditis, retinal detachment, and secondary glaucoma may lead to blindness. Although female terriers, Poodles, and other toy breeds of dogs are predisposed, both sexes of any breed can be affected. Histologically, GME is characterized by the proliferation of lymphocytes, plasma cells, and neutrophils originating from adventitial cells of blood vessels, meninges, and microglia. There may be a proliferation of mononuclear cells around blood vessels which can coalesce and lead to the formation of granulomas (Figure 1).
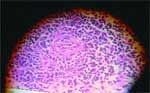
Figure 1: Granulomatous meningoencephalitis. Perivascular cuff of mononuclear cells.
Involvement of any locations in the central nervous system (CNS) has been described, either in a diffuse or focal form. Lesions are found mainly in the white matter, although the gray matter and meninges may also be affected. Cerebrospinal fluid analysis may reveal an increased protein level with or without white blood cells of a mixed cell population (Figure 2).
Focal or multifocal abnormalities may be seen on MR or CT imaging. A definitive diagnosis of GME requires a histopathological examination of the lesion(s) via biopsy, though working diagnosis by exclusion of known infectious causes is often the practical reality.
Poor to grave prognosis
Although corticosteroid therapy may delay the progression of the disease, GME has historically been considered to have a poor to grave prognosis.
In 1998, Munana and Luttgen (J. Am. Vet. Med. Assoc. 1902-1906) reported in a retrospective study that the mean survival time for all dogs with GME was 14 days (range one to 1,215 days). Patients with focal forebrain signs survived longer than those with multifocal signs. Response to therapy with glucocorticosteroids was variable, and in most cases, signs recurred quickly after a brief and partial improvement. Better results were apparently obtained with radiation therapy for focal GME.
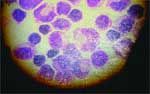
Figure 2: Lymphocytes, monocytes, and macrophages in the cerebrospinal fluid cell of a dog with granulomatous meningoencephalomyelitis.
However, radiation therapy is not helpful for diffuse GME. Financial considerations, multiple anesthesias, and side effects associated with radiation therapy make this type of treatment relatively less common.
Some hope
In a recent conference, PA Cuddon and JR Coates (Proceedings, 20th ACVIM Forum, 319-321) describe new adjunctive and/or alternative therapies for GME that may be effective.
The authors evaluated the effects of cytosine arabinoside and procarbazine in dogs with GME. Cytosine arabinoside (Cytosar-U®) known as ara-C, acts on mitotically active cells by inserting into DNA molecules causing premature chain termination. Ara-C has the ability to cross the blood brain barrier and has immunosuppressive effects. The author study demonstrated that the high dose administration of ara-C is associated with cytotoxic concentrations within the CNS. They recommend a subcutaneous injection of ara-C at a dose of 50 mg/meters2 twice daily for two consecutive days (2003 cost ² $ 6 per 5 ml solution @ 20 mg/ml). This regimen is repeated initially every three weeks. Myelosuppression may occur.
A CBC should be performed 10-14 days after the first course of cytosine therapy, and then once every two to three months. For better therapeutic results, cytosine therapy is usually combined with prednisone (1 mg/kg orally, BID). Prednisone is reduced after the second round of cytosine injections. Cytosine has been occasionally used as a sole agent in some dogs with GME.
Procarbazine (Matulane®) is a monoamine oxidase inhibitor that alkylates DNA and affects RNA and protein synthesis. This substance also crosses the blood brain barrier and shows promise in the treatment of GME.
Due to the small size of dogs with GME, procarbazine should be compounded into a liquid. The authors recommend a 10 mg/ml oil based liquid (the expiration date is 30 days). Procarbazine is administered orally at a dose of 25 to 50 mg/meters2/day. Procarbazine can sometimes cause neurotoxicity, but myelosuppression (thrombocytopenia, leukopenia) seems to be the major limiting factor.
The CBC should be checked once a week for the first month, then monthly thereafter. After the first month of treatment, the authors suggest reducing the frequency of the dose to every other day. The use of these potent medications may also be considered to reduce side effects in dogs particularly sensitive to corticosteroid therapy.