Canine blastomycosis: A review and update on diagnosis and treatment
Blastomycosis is a systemic fungal infection caused by the dimorphic fungus Blastomyces dermatitidis. The infective form of the organism, the mycelial phase, is most likely to be found in sandy, acidic soil near bodies of fresh water.
Blastomycosis is a systemic fungal infection caused by the dimorphic fungus Blastomyces dermatitidis. The infective form of the organism, the mycelial phase, is most likely to be found in sandy, acidic soil near bodies of fresh water.1-5 High organic matter content in the soil from decaying wood byproducts or animal waste together with moist conditions promotes growth of the organism.1 Blastomyces dermatitidis has a relatively wide distribution in North America, including the Mississippi, Missouri, and Ohio river valleys; the Middle Atlantic states; southern Saskatchewan; Manitoba; Quebec; and Ontario.1,4,6 Blastomycosis is most commonly diagnosed in dogs and people.1

Paul Petersen
RISK FACTORS
Dogs at greatest risk for developing clinically apparent blastomycosis are 2- to 4-year-old intact male large-breed dogs living in endemic regions.1,2,3,5 This group of dogs has a greater tendency to roam and to sniff and dig in the soil, resulting in greater exposure to the organism. Sporting dogs and hound breeds are predisposed, most likely because of increased exposure to high-risk areas during hunting.4,5 Residence near a river or lake and access to recently excavated sites have been demonstrated to increase the risk of infection.7,8 Most cases of canine blastomycosis are diagnosed in late summer or early fall.2,5
ROUTE OF INFECTION
Infection most commonly occurs after inhaling spores from contaminated soil.1-3 At normal canine body temperature, the organism transforms to a yeast that can infect the lungs and spread systemically. Although infection almost always begins in the lungs before being disseminated through hematogenous or lymphatic routes to other body tissues, lung lesions occasionally resolve by the time infection in other sites becomes apparent.1-3 The most common sites of clinically apparent infection in dogs include the lungs, lymph nodes, eyes, skin, and bone.1,2,4,9 Subclinical or spontaneously resolving infection is uncommon.1
DIAGNOSIS
Clinical findings in dogs with blastomycosis reflect the systemic inflammatory response and the site or sites of infection. Complete blood count and serum chemistry abnormalities are usually nonspecific and reflect chronic inflammation. Thoracic imaging should be done in all dogs with suspected blastomycosis. Cytologic or histologic examination of infected tissues may reveal the organisms. Serologic, urinary antigen, and polymerase chain reaction (PCR) tests are also available.
Clinical signs
Nonspecific signs of illness, including anorexia, weight loss, and lethargy, are common, and fever (temperature > 103 F [39.4 C]) is present in 40% to 60% of infected dogs.1
Lungs. Lung pathology occurs in 65% to 85% of cases, often resulting in exercise intolerance, cough, tachypnea, cyanosis, or respiratory distress.1-4,9 Lung lesions may also be clinically silent, so a thoracic radiographic examination is recommended in all dogs suspected of having blastomycosis.1
Lymph nodes. Enlargement of one or more peripheral lymph nodes occurs in 30% to 50% of infected dogs, reflecting either reactive hyperplasia or infection of the lymph node by Blastomyces organisms with resultant pyogranulomatous inflammation.1,2,10,11
Eyes. Ocular lesions are identified in 20% to 50% of cases, with endophthalmitis being the most common abnormality.1,12,13 Rapid diagnosis and treatment are essential to preserve vision, so aggressively investigate whether blastomycosis is present in all dogs from endemic regions with early signs of uveitis, including conjunctivitis, iridial hyperemia, aqueous flare, and miosis. Glaucoma secondary to iridocorneal angle obstruction may occur. Other ocular manifestations of blastomycosis may include corneal edema, chorioretinitis, optic neuritis, serous or granulomatous retinal detachment, hyalitis, and vitreal hemorrhage.1-4,12 Panophthalmitis with associated orbital and periorbital inflammation may also develop (Figure 1).1-4,12
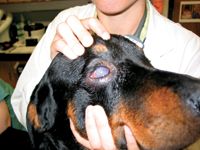
Figure 1. Panophthalmitis in a dog with ocular blastomycosis.
Skin. Dermatologic manifestations of blastomycosis occur in 30% to 50% of infected dogs. Granulomatous proliferative masslike lesions and ulcerated skin lesions draining serosanguineous or purulent fluid are most common.1-4,11 Skin lesions most commonly involve the nasal planum, face, and nail beds.1-3,14,15 A thorough examination of the skin is recommended in all dogs suspected of having blastomycosis, as aspirates or impression smears from seemingly insignificant skin lesions could yield a rapid diagnosis.
Bone. Solitary bone infections causing lameness occur in up to 30% of infected dogs,1-3 typically involving bones of the distal limbs. Radiographically, the fungal osteomyelitis lesions are osteolytic with periosteal proliferation and soft tissue swelling, requiring cytology or biopsy to distinguish between fungal and neoplastic disease.
Other sites. Tissues less commonly infected include the prostate, kidneys, testes, joints, nasal passages, and brain. Central nervous system (CNS) infections are identified in only 3% to 6% of cases.2,3,16,17 Reported neurologic findings include depressed mentation, lethargy, neck pain, circling, cranial nerve deficits, head pressing, seizures, hypermetria, ataxia, and tetraparesis.4,9,18,19 Dogs with CNS blastomycosis nearly always have clinically apparent involvement of extraneural sites, making diagnosis relatively straightforward.3,6,16,18,19
Laboratory findings
Mild normocytic, normochromic, nonregenerative anemia is common, as is moderate leukocytosis characterized by mature neutrophilia or neutrophilia with a left shift. Hypoalbuminemia (75% of cases) and hyperglobulinemia (50% of cases) also occur.1,3,20,21 Albumin, a negative acute phase protein, is frequently decreased with inflammatory conditions. Hypercalcemia has been identified in 2% to 10% of dogs with blastomycosis, perhaps related to active vitamin D production by stimulated macrophages.1,3,20,21
Thoracic radiography
Perform a thoracic radiographic examination in all dogs with suspected blastomycosis, regardless of whether respiratory signs are evident.1 Diffuse miliary to nodular interstitial and bronchointerstitial pulmonary changes are most common (Figures 2A & 2B).22,23 Less often, lung lobe consolidation or a solitary mass within the lung parenchyma is identified (Figure 3).22 Hilar lymphadenopathy may occasionally be evident.1-4
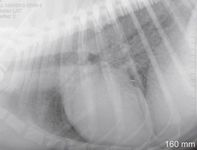
Figure 2A. A lateral thoracic radiograph showing a diffuse miliary interstitial pattern in the lung of a dog with blastomycosis.
The lungs will appear normal radiographically in a few dogs with pulmonary parenchymal disease since inflammatory nodules smaller than 5 mm in diameter may not be detected.24,25 Obtain right and left lateral views and a ventrodorsal radiograph whenever possible in a stable patient to increase the likelihood of finding small lesions.26 Computed tomography is more accurate in detecting pulmonary nodules in people than radiography is and may be beneficial in dogs with subtle pulmonary lesions.25
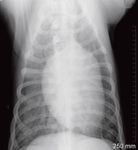
Figure 2B. A ventrodorsal thoracic radiograph showing a diffuse miliary interstitial pattern in a dog with blastomycosis.
Cytologic diagnosis
Blastomycosis is most reliably diagnosed by demonstrating the organism in cytologic or histologic samples from infected tissues. Samples from infected sites usually show evidence of pyogranulomatous or purulent inflammation, which should prompt a careful search for yeast cells.11
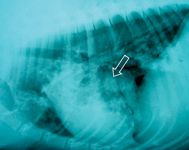
Figure 3. A lateral thoracic radiograph showing a focal granuloma (arrow) in the lung of a dog with blastomycosis.
Lymph nodes. Cytologic evaluation of fine-needle aspirates from enlarged, infected lymph nodes yields a diagnosis in 67% to 82% of cases (Figure 4).3,11 Aspirates from normal-size lymph nodes will also occasionally reveal organisms, so lymph node fine-needle aspiration is recommended in all dogs with suspected blastomycosis.24
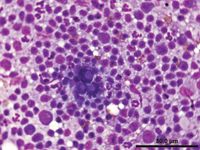
Figure 4. Cytologic examination of a fine-needle aspirate from an enlarged lymph node in a dog with blastomycosis reveals Blastomyces organisms (Wright's-Giemsa stain).
Skin. When skin lesions are present, cytologic evaluation of exudates or aspirates from dermal lesions yields positive results in 85% to 94% of cases.3,11
Lungs. Cytologic examination of samples obtained from the lungs by transtracheal aspiration, bronchoalveolar lavage, and transthoracic lung aspiration has been evaluated in the diagnosis of pulmonary blastomycosis.9,10,27,28 Although early reports suggested that B. dermatitidis was not likely to be identified in transtracheal wash samples, two recent retrospective studies have demonstrated the organisms in 69% and 76% of transtracheal wash samples from dogs with radiographically evident pulmonary blastomycosis (Figure 5).9,27 Bronchoalveolar lavage yields similar results and generally superior sample cellularity than transtracheal wash does but requires general anesthesia.27 Transthoracic fine-needle aspiration of solitary or diffuse pulmonary lesions yields a diagnosis in about 80% of dogs with pulmonary blastomycosis but occasionally requires acquiring and evaluating multiple samples.10,28 The diagnostic utility of cytologic evaluation of respiratory samples obtained from patients with blastomycosis but without radiographically apparent lung disease has not been evaluated.
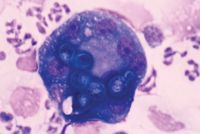
Figure 5. Blastomyces organisms have been phagocytosed by macrophages in this transtracheal wash sample from a dog with pulmonary blastomycosis. Pyogranulomatous inflammation is evident (Wright's-Giemsa stain; 100X magnification).
Eyes. Most dogs with ocular blastomycosis have concurrent lung, skin, or lymph node involvement, allowing cytologic diagnosis to be made based on samples collected less invasively from these tissues. Vitreal aspirates and subretinal aspirates have been recommended in patients with only ocular involvement, but these techniques may threaten vision in an eye that is not already blind.1,11,12 Cytologic evaluation of vitreal aspirates resulted in organism identification in 100% of five cases in one report (Figure 6).3
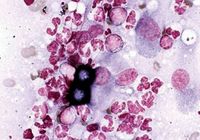
Figure 6. Cytologic examination of a vitreal aspirate from a dog with ocular blastomycosis reveals pyogranulomatous inflammation and Blastomyces organisms (Wright's-Giemsa stain; 50X).
CNS. Definitive diagnosis based on cerebrospinal fluid (CSF) analysis is rarely possible in dogs with blastomycosis involving the brain or spinal cord.16,18,19 Analysis of CSF from dogs with CNS blastomycosis typically reveals a lymphocytic to mixed pleocytosis, with a neutrophil concentration ranging from 5% to 40% of CSF leukocytes.16,18,29 But, occasionally, CSF will be normal or markedly neutrophilic.10 Blastomyces organisms are almost never identified in CSF.16,18,29 However, CNS blastomycosis rarely occurs in isolation, and cytologic identification of organisms in other infected tissues allows blastomycosis to be diagnosed.2,3,9,16,18,29
Serologic testing
When cytologic samples from infected tissues are not diagnostic yet you highly suspect blastomycosis, serologic testing may be performed. Dogs with blastomycosis produce antibodies directed against the Wisconsin-1 (WI-01) and A antigens of B. dermatitidis.4
The agar gel immunodiffusion test for antibodies against the Blastomyces A antigen is the most commonly used serologic test and has a reported sensitivity of 40% to 90% and specificity of 90% to 100%.1,2,4,27 The test results are often negative early in the course of infection,2,4,27,30 making it unlikely that the agar gel immunodiffusion test will be useful in dogs without overt systemic blastomycosis. Antibody titers may persist in cured animals, making it impossible to use agar gel immunodiffusion results to monitor response to therapy or disease recurrence.2,30 No published studies have evaluated the duration of persistent positive titers after treatment.
A radioimmunoassay test to detect serum antibodies against the WI-01 antigen has been reported to detect 92% of infected dogs while maintaining 100% specificity, but this test is not commercially available.1,4
Urinary antigen testing
Recently, an enzyme immunoassay test to detect B. dermatitidis antigen in urine has been described (MVista Blastomyces dermatitidis Antigen EIA—MiraVista Diagnostics).31-33 This test is reported to be highly sensitive, detecting antigen in urine from 93% of people and up to 100% of dogs with systemic or pulmonary blastomycosis. Cross-reactivity with other fungal agents (especially Histoplasma capsulatum) and a few nonspecific false positive results have been reported.31-33
PCR testing
A few veterinary laboratories offer PCR-based diagnostic tests for fungal diseases. The South Dakota Animal Disease Research & Diagnostic Laboratory and HealthGene offer veterinary-specific tests for Blastomyces species. PCR promises increased sensitivity and speed of diagnosis compared with conventional diagnostic tests, but the utility of PCR in early diagnosis of canine blastomycosis has not been evaluated.30 For now, PCR tests should be used as supportive evidence to complete a clinical picture rather than as a sole method of diagnosis.
THERAPY
Drugs most often recommended to treat dogs with blastomycosis are amphotericin B and the azole antifungals, including itraconazole, ketoconazole, fluconazole, and voriconazole. Table 1 provides a summary of recommended dosages for commonly used antifungal agents. These drugs are not FDA-approved for veterinary use.

Table 1. Drugs and Dosages for Treating Canine Blastomycosis
Amphotericin B
Amphotericin B is a polyene antibiotic that binds ergosterol, an essential component of the fungal cell wall, thereby disrupting the cell wall and causing the organism's death. Amphotericin B's principal adverse effect is cumulative nephrotoxicosis, so monitor renal function before each dose.4,11,34
The desoxycholate form of amphotericin B is most commonly administered as an intravenous infusion. Sodium loading before treatment (20 ml/kg of 0.9% sodium chloride solution administered intravenously over 60 minutes) and slow intravenous administration (over five or six hours) of the amphotericin B dose (0.5 mg/kg) diluted in 5% dextrose in water solution or 2.5% dextrose in 0.45% saline solution (500 ml for dogs < 20 kg; 1,000 ml for dogs > 20 kg) may decrease the nephrotoxicity of this formulation.2,34 Alternatively, the amphotericin B dose can be diluted in 2.5% dextrose in 0.45% saline solution (500 ml for dogs < 20 kg; 1,000 ml for dogs > 20 kg) and administered subcutaneously.35 Most protocols require that the amphotericin B be administered every other day until a cumulative dose of 8 to 10 mg/kg has been achieved or until renal function deteriorates.1,34 A cumulative dose of 4 to 6 mg/kg may be adequate when amphotericin B is administered together with an azole.
Amphotericin B may be administered during the initial treatment of dogs with severe or rapidly progressive blastomycosis to improve the rate of recovery. Combination therapy with amphotericin B and an azole antifungal allows for a decreased total dose of amphotericin B to be administered, potentially decreasing the potential for nephrotoxicity.
A lipid-complexed formulation of amphotericin B has recently become available. This formulation, Abelcet (The Liposome Company), is eight to 10 times less nephrotoxic than amphotericin B desoxycholate, allowing higher cumulative doses to be administered.2,12,34,36 The higher administered dose and increased uptake of lipid complexes by phagocytic cells of the reticuloendothelial system may improve efficacy with this formulation.36 Dilute Abelcet in 5% dextrose in water to a concentration of 1 mg/ml, and administer to dogs at a dose of 1 to 3 mg/kg as a one- to two-hour intravenous infusion every other day until a cumulative dose of 24 to 27 mg/kg has been achieved.
Azole antifungals
The azole antifungals ketoconazole, itraconazole, fluconazole, and voriconazole interfere with ergosterol synthesis. Ketoconazole is less expensive than the other azole antifungals, but treating canine blastomycosis with ketoconazole alone results in disease resolution in < 50% of cases and is associated with a high rate of relapse and adverse effects.2,17,37
Itraconazole. Itraconazole is the azole most often recommended for treating dogs with blastomycosis since it is as effective as amphotericin B but is associated with fewer adverse effects and can be administered orally.1,4,17,34,37 Itraconazole is a weak base that is best absorbed when administered with food. Peak plasma concentrations do not occur until six to 14 days after treatment is begun, resulting in some lag time before clinical response. Itraconazole is extensively bound to plasma proteins and is lipophilic, leading to good distribution throughout most tissues, but concentrations in urine and CSF are low. Itraconazole does not cross the normal blood-brain, blood-prostate, or blood-ocular barrier, but infections with Blastomyces organisms at these sites often respond well to treatment with itraconazole, perhaps because of increased penetration of the drug when inflammation is present.34,36 Itraconazole is typically administered at a dose of 5 mg/kg orally twice a day for five days, followed by 5 mg/kg once daily, or divided twice daily, for the remainder of the treatment period.1,2,17,34
Most dogs receiving 5 mg/kg/day of itraconazole do not develop any clinical signs of toxicosis. Asymptomatic increases in alanine transaminase (ALT) activity are common but should not prompt a change in therapy unless the dog exhibits anorexia, vomiting, or abdominal pain.1,34 Most gastrointestinal side effects are dose-related and will resolve if the drug is discontinued until the dog's appetite returns and then reinstituted at half the former dose.1,34
Vasculitis resulting in ulcerative dermatitis or limb swelling develops in 7.5% of dogs receiving a high dose of itraconazole (10 mg/kg/day) but does not develop in dogs receiving 5 mg/kg/day. Be sure to distinguish this drug reaction from a manifestation of cutaneous blastomycosis. Reducing the daily-administered dose of itraconazole to 5 mg/kg usually results in rapid healing of the ulcerated lesions and resolution of limb edema.17
Fluconazole. Fluconazole is an azole antifungal that is minimally protein bound and highly water soluble, penetrating the blood-brain, blood-prostate, and blood-ocular barriers well and achieving high concentrations in urine, CSF, and ocular fluids.4,23,24 Fluconazole (2.5 to 5 mg/kg orally or intravenously twice a day) may have a role in treating CNS, prostatic, and urinary blastomycosis, but itraconazole is more effective in most dogs with blastomycosis.4,37
Voriconazole. Voriconazole is a derivative of fluconazole that has a broader spectrum of action, improving its efficacy against blastomycosis while maintaining wide tissue distribution and the ability to cross the blood-brain, blood-ocular, and blood-prostate barriers. As such, this drug may be effective in treating CNS fungal infections including blastomycosis. The dose in dogs has not been well-established, but 5 to 10 mg/kg orally or intravenously twice a day has been recommended.
DURATION OF THERAPY AND RESPONSE TO TREATMENT
About 70% to 80% of dogs with blastomycosis respond completely to treatment with itraconazole, amphotericin B, or the two drugs in combination.1,2,17,37 When the drugs are used in combination, they are administered simultaneously initially, and then the itraconazole is continued as a sole agent once the desired cumulative dose of amphotericin B has been administered.
Administer itraconazole or the other azoles for at least 60 days in all infected dogs, and continue for at least one month after all clinical and radiographic evidence of disease has resolved.12,17 Treat dogs with severe lung involvement for at least 90 days. Even with appropriate duration of treatment, 20% to 25% of dogs will relapse after itraconazole therapy. The likelihood of relapse is related to the severity of the initial lung disease, and it most often occurs within six months of therapy cessation.1 Retreatment with an additional 60- to 90-day course of itraconazole has an 80% chance of producing a cure.1
PROGNOSIS
The two most important negative prognostic factors are CNS involvement and severe lung disease.1,4,17 Most dogs with brain involvement will die, but aggressive treatment with amphotericin B, fluconazole, or voriconazole may occasionally be effective.1
Dogs with severe diffuse pulmonary blastomycosis often deteriorate during the first two or three days of treatment, perhaps related to an inflammatory response directed against dying organisms in the lungs.1,2,17 Fifty percent of these dogs will die within the first seven days of therapy.1 Anti-inflammatory dosages of dexamethasone (0.25 mg/kg intravenously once a day for two or three days) should be routinely administered to dogs with severe pulmonary infiltrates that develop worsening respiratory distress during antifungal therapy.1 Dogs that receive glucocorticoids and recover should then be treated for at least 90 days with antifungal drugs.
Although itraconazole does not normally achieve high concentrations in the eye, the response to treatment with a high itraconazole dose (5 mg/kg b.i.d.) in dogs with mild posterior segment disease without complete retinal separation has been good, with most dogs being cured and retaining vision in the affected eyes.13,38,39 Blind eyes that are severely affected with glaucoma or endophthalmitis are unlikely to become visual and should probably be enucleated to prevent them from serving as a persistent focus of infection.12,14,15,40
PUBLIC HEALTH CONCERNS
At 98.6 F (37 C) or above, B. dermatitidis exists in a yeast phase that is too large to be transmitted by coughing to other dogs or to people as an aerosol. Cutaneous inoculation through needle sticks or wound contamination has, however, been reported.1,4 The organism's mycelial phase may form in bandaged skin lesions4 and may aerosolize and infect people present during bandage changes. Draining tracts or ulcerative lesions should, thus, be left uncovered, and staff members should wear protective clothing including gloves, gowns, and surgical masks when in contact with patients with these lesions. Culture of B. dermatitidis should only be attempted in laboratories with proper facilities, and samples should be clearly marked to minimize risk to laboratory personnel4 since culture promotes growth of the highly infectious mycelial phase.
M. Casey Gaunt, DVM
Susan M. Taylor, DVM, DACVIM
Department of Small Animal Clinical Sciences
Western College of Veterinary Medicine
University of Saskatchewan
Saskatoon, SK S7N 5B4
REFERENCES
1. Legendre AM. Blastomycosis. In: Greene CE, ed. Infectious diseases of the dog and cat. 3rd ed. St. Louis, Mo: Saunders, 2006;569-576.
2. Kerl ME. Update on canine and feline fungal diseases. Vet Clin North Am Small Anim Pract 2003;33(4):721-747.
3. Arceneaux KA, Taboada J, Hosgood G. Blastomycosis in dogs: 115 cases (1980-1995). J Am Vet Med Assoc 1998;213(5):658-664.
4. Brömel C, Sykes JE. Epidemiology, diagnosis, and treatment of blastomycosis in dogs and cats. Clin Tech Small Anim Pract 2005;20(4):233-239.
5. Rudmann DG, Coolman BR, Perez CM, et al. Evaluation of risk factors for blastomycosis in dogs: 857 cases (1980-1990). J Am Vet Med Assoc 1992;201(11):1754-1759.
6. Harasen GLG, Randall JW. Canine blastomycosis in Southern Saskatchewan. Can Vet J 1986;27(10):375-378.
7. Baumgardner DJ, Paretsky DP, Yopp AC. The epidemiology of blastomycosis in dogs: north central Wisconsin, USA. J Med Vet Mycol 1995;33(3):171-176.
8. Baumgardner DJ, Steber D, Glazier R, et al. Geographic information system analysis of blastomycosis in northern Wisconsin, USA: waterways and soil. Med Mycol 2005;43(2):117-125.
9. McMillan CJ, Taylor SM. Transtracheal aspiration in the diagnosis of pulmonary blastomycosis (17 cases: 2000-2005). Can Vet J 2008;49(1):53-55.
10. Crews LJ, Feeney DA, Jessen CR, et al. Utility of diagnostic tests for and medical treatment of pulmonary blastomycosis in dogs: 125 cases (1989-2006). J Am Vet Med Assoc 2008;232(2):222-227.
11. Garma-Aviña A. Cytologic findings in 43 cases of blastomycosis diagnosed ante-mortem in naturally-infected dogs. Mycopathologia 1995;131(2):87-91.
12. Krohne SG. Canine systemic fungal infections. Vet Clin North Am Small Anim Pract 2000;30(5):1063-1090.
13. Bloom JD, Hamor RE, Gerding PA Jr. Ocular blastomycosis in dogs: 73 cases, 108 eyes (1985-1993). J Am Vet Med Assoc 1996;209(7):1271-1274.
14. Legendre AM. Blastomycosis. In Greene CE, ed. Infectious diseases of the dog and cat. 1st ed. Philadelphia, Pa: WB Saunders, 1990;669-678.
15. Legendre AM. Blastomycosis. In Greene CE, ed. Infectious diseases of the dog and cat. 2nd ed. Philadelphia, Pa: WB Saunders, 1998;371-377.
16. Lavely J, Lipsitz D. Fungal infections of the central nervous system in the dog and cat. Clin Tech Small Anim Pract 2005;20(4):212-219.
17. Legendre AM, Rohrbach BW, Toal RL, et al. Treatment of blastomycosis with itraconazole in 112 dogs. J Vet Intern Med 1996;10(6):365-371.
18. Nafe LA, Turk JR, Carter JD. Central nervous system involvement of blastomycosis in the dog. J Am Anim Hosp Assoc 1983;19:933-936.
19. Saito M, Sharp NJH, Munana K, et al. CT findings of intracranial blastomycosis in a dog. Vet Radiol Ultrasound 2002;43(1):16-21.
20. Dow SW, Legendre AM, Stiff M, et al. Hypercalcemia associated with blastomycosis in dogs. J Am Vet Med Assoc 1986;188(7):706-709.
21. Crews LJ, Sharkey LC, Feeney DA, et al. Evaluations of total and ionized calcium status in dogs with blastomycosis: 38 cases (1997-2006). J Am Vet Med Assoc 2007;231(10):1545-1549.
22. Crews LJ, Feeney DA, Jessen CR, et al. Radiographic findings in dogs with pulmonary blastomycosis: 125 cases (1989-2006). J Am Vet Med Assoc 2008;232(2):215-221.
23. Karnik PS. What is your diagnosis? Diffuse nodular bronchointerstitial pattern. J Am Vet Med Assoc 2003;222(1):27-28.
24. Gaunt MC, Taylor SM, Kerr ME. Central nervous system blastomycosis in a dog. Can Vet J 2009;in press.
25. Nemanic S, London CA, Wisner ER. Comparison of thoracic radiographs and single breath-hold helical CT for detection of pulmonary nodules in dogs with metastatic neoplasia. J Vet Intern Med 2006;20(3):508-515.
26. Ober CP, Barber D. Comparison of two- vs. three-view thoracic radiographic studies on conspicuity of structured interstitial patterns in dogs. Vet Radiol Ultrasound 2006;47(6):542-545.
27. Hawkins EC, DeNicola DB. Cytologic analysis of tracheal wash specimens and bronchoalveolar lavage fluid in the diagnosis of mycotic infections in dogs. J Am Vet Med Assoc 1990;197(1):79-83.
28. Wood EF, O'Brien RT, Young KM. Ultrasound-guided fine-needle aspiration of focal parenchymal lesions of the lung in dogs and cats. J Vet Intern Med 1998;12(5):338-342.
29. Friedman JA, Wijdicks EFM, Fulgham JR, et al. Meningoencephalitis due to Blastomyces dermatitidis: case report and literature review. Mayo Clin Proc 2000;75(4):403-408.
30. Dial SM. Fungal diagnostics: current techniques and future trends. Vet Clin North Am Small Anim Pract 2007;37(2):373-392.
31. Durkin M, Witt J, LeMonte A, et al. Antigen assay with the potential to aid in diagnosis of blastomycosis. J Clin Microbiol 2004;42(10):4873-4875.
32. Shurley JF, Legendre AM, Scalarone GM. Blastomyces dermatitidis antigen detection in urine specimens from dogs with blastomycosis using a competitive binding inhibition ELISA. Mycopathologia 2005;160(2):137-142.
33. Spector D, Legendre AM, Wheat J, et al. Antigen and antibody testing for the diagnosis of blastomycosis in dogs. J Vet Intern Med 2008;22(4):839-843.
34. Grooters AM, Taboada J. Update on antifungal therapy. Vet Clin North Am Small Anim Pract 2003;33(4):749-758.
35. O'Brien CR, Krockenberger MB, Martin P, et al. Long-term outcome of therapy for 59 cats and 11 dogs with cryptococcosis. Aust Vet J 2006;84(11):384-392.
36. Wiebe V, Karriker M. Therapy of systemic fungal infections: a pharmacologic perspective. Clin Tech Small Anim Pract 2005;20(4):250-257.
37. Taboada J, Grooters AM. Systemic mycoses. In: Ettinger SJ, Feldman EC, eds. Textbook of veterinary internal medicine. 6th ed. Vol. 1. St. Louis, Mo: Elsevier Saunders 2005;671-690.
38. Brooks DE, Legendre AM, Gum GG, et al. The treatment of ocular blastomycosis with systemically administered itraconazole. Prog Vet Comp Ophthalmol 1991;4:263-268.
39. Gionfriddo JR, Powell CC. Disseminated blastomycosis with ocular involvement in a dog. Vet Med 2002;97(6):423-431.
40. Howard J, Arceneaux KA, Paugh-Partington B, et al. Blastomycosis granuloma involving the cranial vena cava associated with chylothorax and cranial vena caval syndrome in a dog. J Am Anim Hosp Assoc 2000;36(2):159-161.