A challenging case: Uveitis and secondary glaucoma in a cat
A 9-year-old male neutered domestic shorthaired cat was referred to The Veterinary Eye Clinic in Wheat Ridge, Colo., for evaluation of acute, progressive uveitis in both eyes and glaucoma in the right eye.
A 9-year-old male neutered domestic shorthaired cat was referred to The Veterinary Eye Clinic in Wheat Ridge, Colo., for evaluation of acute, progressive uveitis in both eyes and glaucoma in the right eye. The cat went outside occasionally to hunt. It had no known history of trauma or travel and was receiving no medications, and its vaccination status was current for feline viral rhinotracheitis, calicivirus infection, panleukopenia, and rabies.

Vital Stats
HISTORY
One month before referral, the cat had been presented to an emergency clinic because of acute hyphema in the right eye and uveitis in both eyes. The cat was mildly lethargic, and its temperature was 102.4 F. The intraocular pressure (IOP) was 16 mm Hg in both eyes (normal = 15 to 35 mm Hg).1 Trauma was suspected, and one drop of prednisone acetate was prescribed for use in both eyes four times a day.
The referring veterinarian saw the cat for a recheck examination two days later, and the IOP was 45 mm Hg in the right eye and 5 mm Hg in the left eye. Hyphema had developed in the left eye, and hypopyon was present in the right eye. Neither eye had fluorescein stain uptake. The cat had also become more lethargic and exhibited inappetence.
A serum chemistry profile had revealed a high normal globulin concentration (5.4 g/dl; reference range = 2.8 to 5.4 g/dl). A complete blood count had shown a mild lymphocytosis (9,860/µl; reference range = 400 to 6,800/µl). The cat's blood pressure was normal, and a thoracic radiographic examination revealed no abnormalities. The results of feline immunodeficiency virus (FIV) and feline leukemia virus (FeLV) tests (SNAP FIV/FeLV Combo—Idexx) were negative.
Trauma, infectious disease, or idiopathic uveitis with secondary glaucoma were the top differential diagnoses. Treatment prescribed by the referring veterinarian included intravenous mannitol (1 g/kg) administered over 30 minutes and subcutaneous triamcinolone acetonide (0.25 mg/kg given once). A topical carbonic anhydrase inhibitor, dorzolamide (one drop in each eye twice a day), and oral prednisolone (1 mg/kg once a day) were also prescribed, and the cat was discharged.
Over the next 20 days, the IOP in the right eye decreased and the cat's ocular pain seemed to diminish. However, on a recheck 21 days after initial presentation, the IOP was 37 mm Hg in the right eye and 8 mm Hg in the left eye. The patient had become blind in right eye and had bilateral uveitis. The pupils had become irregularly shaped because of posterior synechiae (adhesions between the iris and the anterior lens capsule).2 The cat also exhibited inappetence. The cat was then referred to a veterinary ophthalmologist.
OCULAR EXAMINATION
The initial ocular examination at The Veterinary Eye Clinic revealed that the right eye was blind and had buphthalmos with iris bombé and posterior synechiae (Figure 1). (Iris bombé occurs when there is forward bowing of the iris and synechiae that seal the iris to the lens so aqueous humor cannot pass through the pupil into the anterior chamber.) The base of the iris had obstructed the filtration angle because of forward bowing of the iris, causing obstructive glaucoma.3 Fibrin and a blood clot were present in the right pupil (Figure 2). Ulcerative keratitis had developed, likely from exposure, in the right eye. Pars planitis (inflammation of the posterior portion of the ciliary body) was also evident.

1. The cat's right eye exhibited buphthalmos, and the left pupil was irregularly shaped.
Uveitis, evidenced by low IOP, was present in the left eye. The left eye also had posterior synechiae and a blood clot in the pupil. The fundus could not be visualized in either eye.
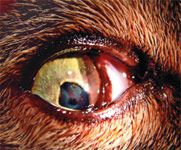
2. The cat's right eye had anterior synechiæ and fibrin in the pupil.
The rest of the physical examination findings were normal, and the cat weighed 11.9 lb (5.4 kg).
DIFFERENTIAL DIAGNOSES
Differential diagnoses for uveitis include trauma, systemic infection, neoplasia, and immune-mediated causes. Coagulopathies and systemic hypertension can also cause hyphema.
The most common intraocular primary neoplasms are iris melanoma or ciliary body adenocarcinoma.1 Ocular neoplasia can also result from metastatic cancer, most commonly lymphosarcoma.
Viral causes of uveitis in cats include feline infectious peritonitis (FIP), FeLV, and FIV infections. Cryptosporidiosis, blastomycosis, histoplasmosis, coccidioidomycosis, and, rarely, candidiasis should be considered depending on the region.4 The algae Prototheca species can also cause uveitis.1 Bacterial causes include Bartonella, Mycoplasma, and Ehrlichia species.4 Toxoplasmosis is a common protozoal cause, which often leads to posterior segment disease, including chorioretinitis and pars planitis.5 Bartonella henselae and herpesvirus infection have also more recently been implicated as causing uveitis.6 While characteristic signs of specific infectious diseases exist (e.g. pars planitis and chorioretinitis caused by toxoplasmosis), studies have shown that intraocular changes are often indistinguishable among various infectious diseases.7
Specific immune-mediated diseases in the eye include lens-induced uveitis (phacoclastic uveitis), Vogt-Koyanagi-Harada-like syndrome, and immune-mediated vasculitis. Immune-mediated causes tend to be a diagnosis of exclusion. However, many systemic inflammatory responses (including systemic infection) can also lead to a secondary immune-mediated uveitis. For example, no infectious agents may be identified in the aqueous humor after anterior chamber paracentesis, but uveitis is still present because of systemic infection and activated T cells that have migrated back to the eye.8
A systemic infection was suspected in this cat because there was no known history of trauma, the ocular disease had progressed, and the cat was lethargic and inappetent. The differential diagnoses were bartonellosis, toxoplasmosis, and FIP, FIV, or FeLV infection (although the test results for FIV and FeLV were negative). Other infectious agents were less likely since the cat lived in Colorado9 and had no history of travel.
The cat had no intraocular mass and no blood work or thoracic radiograph abnormalities that suggested neoplasia. Because the uveitis was bilateral and the patient had mild lethargy and lymphocytosis, the cause was likely to be idiopathic, immune-mediated, or a manifestation of a systemic disease such as an infection.
DIAGNOSTIC TESTS
The patient was anesthetized, and anterior chamber paracentesis was performed. Atraumatic tissue forceps were used to grasp the bulbar conjunctiva, and a 27-ga needle was inserted through the perilimbal conjunctiva and limbal cornea to aspirate a small amount of aqueous humor.1 PCR testing was done on the aspirate to evaluate for fip infection and bartonellosis.
In addition, serum was submitted for protein electrophoresis to distinguish between a monoclonal and polyclonal gammopathy as a cause of the cat's elevated globulin concentration. A polyclonal gammopathy is expected in patients with inflammatory or infectious conditions, especially chronic infections, toxoplasmosis, or FIP infection. A monoclonal gammopathy is consistent with some types of neoplasia, such as multiple myeloma. Serum was also submitted to evaluate the cat's antibody titer against Toxoplasma species.
DIAGNOSIS
The results of aqueous humor PCR testing for bartonellosis were negative. The results of serum assays for antibodies against Toxoplasma species were IgG = 1:64 and IgM = 1:128. An IgM titer > 1:64 is consistent with recent exposure and active infection.9 Serum electrophoresis showed a normal distribution of the immunoglobulins. The mild elevation in serum globulins was likely due to antigenic stimulation or inflammation.
TREATMENT
Ten days later, the cat was presented to the ophthalmologist for a recheck examination. Progressive buphthalmos was noted in the right eye. Laser diode iridotomy was performed while the cat was anesthetized to control the IOP and for cosmetic reasons. Multiple holes were created at two-thirds the distance from the pupil to the base of the iris to deflate the iris bombé, allowing the iris to collapse away from the filtration angle,10 which enabled aqueous humor to flow out through the filtration angle and avoid worsening glaucoma.
The cat was treated for systemic toxoplasmosis with azithromycin (10 mg/kg orally once a day for seven days and then every other day for six weeks).11 An oral corticosteroid was also needed to control the arachidonic acid pathway and decrease permeability of the blood-ocular barrier to control uveitis and secondary glaucoma.12 Thus, an anti-inflammatory dose of prednisolone (0.5 mg/kg) was given orally once daily and was tapered over six weeks.
A topical nonsteroidal anti-inflammatory drug (NSAID), diclofenac 0.1% ophthalmic solution, was also administered initially four times a day and then twice a day in both eyes. A bacitracin-neomycin-polymyxin B ophthalmic ointment was administered four times a day to help prevent an infection in the ulcer in the right eye. The ulcer was likely a result of exposure keratitis secondary to buphthalmos. Avoiding infection is imperative because infected corneal ulcers can progress rapidly with stromal degeneration and corneal perforation, some in as little as 24 hours.13
The cat was discharged the same day that laser diode iridotomy was performed. Because the cat had clinical signs of toxoplasmosis two months earlier, it was highly unlikely that it was still shedding oocysts.14 The clients were advised that the best ways to avoid toxoplasmosis were to scoop cat feces daily before sporulation could occur and to avoid eating raw meat. It was also recommended that the cat not be allowed to hunt.14
FOLLOW-UP
IOPs were evaluated weekly, revealing a trend toward normal pressure. A month after referral to the ophthalmologist, the IOPs in both eyes were 14 mm Hg. The iris bombé had resolved in the right eye. However, the right eye remained nonvisual with a cloudy anterior chamber. The left pupil appeared irregular in shape because of focal regions of posterior synechiae, but it remained visual (Figure 3). The fundi were never able to be clearly visualized.

3. The cat's left eye remains visual but the pupil is irregular in shape because of posterior synechiæ, where portions of the iris have attached to the anterior lens capsule.
Six weeks after initiating the ophthalmologist's treatment protocol, the cat had improved, and all medications were discontinued except for the twice-daily diclofenac ophthalmic drops. Additionally, 40 mg of oral aspirin every third day was added after the prednisolone was tapered. The owners were instructed to monitor for gastrointestinal side effects while the NSAIDs were being administered; none were reported. The cat continues to improve and is eating well and has gained a half pound.
DISCUSSION
Uveitis is inflammation of the uveal tract (the iris, ciliary body, and choroid). The intraocular immune response is unique because the eye is an immunologically privileged site, which means the eye can modify intraocular antigen presentation and control the type and amount of inflammation.8 Immunocompetent lymphocytes migrate back to the eye as a result of activated inflammatory mediators. If the inciting antigen is not removed, chronic inflammation develops because of the immunocompetent lymphocytes in the uvea.2 Because of this pathophysiology, uveitis can result from systemic infection, autoimmune disease, neoplasia, or trauma.
Aqueous flare is the hallmark of uveitis and is visible when proteins are in the anterior chamber, indicating a breakdown of the blood-ocular barrier. These proteins scatter light, making the aqueous humor appear cloudy. Hyperemia of the conjunctival vessels and aqueous flare are characteristic, but anterior uveitis can also manifest as corneal edema, hyphema, hypopyon, iris color change (usually to a darker shade), keratic precipitates (deposition of inflammatory cells on the corneal endothelium), miosis, and pain.1
Decreased IOP occurs when the ciliary body becomes inflamed, decreasing aqueous humor production and increasing perfusion through the posterior vascular pathway. In acute uveitis, blepharospasm, enophthalmos, and elevation of the third eyelid are common. With chronic uveitis, these manifestations of ocular pain may no longer be present. Patients with chronic uveitis may also present with posterior synechiae. Iris bombé and glaucoma can occur, as seen in this cat.
Work-up for feline uveitis
Always investigate uveitis, as cats with systemic and fatal diseases may present with only uveitis.15 In one study of 124 cats with uveitis, serologic evidence of infectious agents was found in 83% of the samples.16 Other studies indicate that systemic disease is present in 25% to 90% of cats with uveitis.2
Initially test any cat with uveitis of unknown cause for FIV and FeLV infections, and ask the owner about travel, trauma history, vaccination status, and whether the cat goes outdoors. Additional diagnostic tests when systemic causes are suspected include a complete blood count, a serum chemistry profile, and a urinalysis. Fungal antibody titers and antibody titers against Toxoplasma gondii also may be useful. Anterior chamber paracentesis with PCR and culture of the aqueous humor for Bartonella species, coronavirus, T. gondii, and Cryptococcus species are helpful. A Western blot may be performed to look for Bartonella species.5 Even with an exhaustive diagnostic work-up, a definitive cause may not be found.6
This patient had anterior uveitis and developed secondary iris bombé, glaucoma, and finally blindness. This progression illustrates the importance of early recognition and treatment of uveitis. When inflammation compromises the integrity of the blood-ocular barrier, proteins, fibrin, and blood leak into the aqueous humor and the vitreous. Synechiae and glaucoma can follow. Glaucoma can also occur when inflammatory mediators affect the trabecular endothelium and physically obstruct the filtration angle or block aqueous flow through the pupil.2
Treating uveitis
When uveitis is recognized, administer atropine to dilate the pupil to avoid synechiae and administer topical (e.g. diclofenac) and systemic anti-inflammatory medications to avoid complicating sequelae. Anti-inflammatory medications are the mainstay of treatment for uveitis. The treatment goals for any uveitis case are to block prostaglandin formation and other mediators of inflammation, reduce painful ciliary spasm, dilate the pupil to prevent synechiae and secondary glaucoma, and restore the blood-aqueous and blood-retinal barriers.12
Thoughtful use of atropine is important because overuse can decrease tear production and cause glaucoma. The idea is to keep the pupil moving; do not simply administer atropine twice daily but rather wait until the pupil begins to constrict before repeat dosing. Glaucoma results when mydriasis further reduces the outflow of aqueous humor due to the accumulation of red blood cells and inflammatory cells together with a dilated iris.17
Iridotomy
Laser iridotomy was performed in this cat to control the IOP in the right eye. The potential complications associated with iris surgery include hemorrhage, the iridotomy sites resealing, and iris bombé recurrence.10 Gonioscopy was not performed to document that the angles were opened postoperatively; however, the IOP in the right eye stabilized and eventually decreased, and the iris bombé resolved after the procedure was performed. Because the right eye was already blind, these results were considered successful.
Route of T. gondii infection
In this patient, the T. gondii infection was probably acquired by hunting. Toxoplasma gondii is an intracellular protozoan, and cats are the definitive host. Cats become infected by ingesting cysts from the tissue of intermediate hosts (usually rodents).2 After ingestion, encysted bradyzoites transform into tachyzoites and undergo the sexual phase of their life cycle in the feline intestine. This phase occurs when oocysts are shed in the feces.14
People may become infected by ingesting or inhaling the oocysts in cat feces or by ingesting undercooked red meat. Most cats become infected through hunting and will shed oocysts after initial infection for less than three weeks.9 Litter boxes should be cleaned out daily as it takes 24 hours for oocysts to sporulate and become infective. By the time a cat shows signs of toxoplasmosis, it is no longer shedding oocysts.
Prevalence of T. gondii infection and clinical signs
It is estimated that about 30% of people and cats have been exposed to T. gondii in the United States.9 However, immunocompetent people are generally asymptomatic. Studies have shown no direct correlation between human toxoplasmosis and cat ownership.9
Some studies have shown that as many as 74% of cats with uveitis are seropositive for T. gondii depending on the geographic area.18 Uveitis may occur in healthy cats recently exposed to T. gondii; it is unknown why some develop ocular pathology while others do not. The severity of disease is thought to be associated with a host's ability to mount a proper cell-mediated immune response, the severity of the primary infection, and the T. gondii strain.19 Neonatal T. gondii infection can cause transient anterior uveitis and, more commonly, chorioretinitis in kittens; it is unknown what role infection in utero plays in chronic or recurrent uveitis in adult cats.18
Clinical signs of T. gondii infection vary in cats: in one study of 100 cats, 36 had multiorgan involvement, 26 had mostly pulmonary signs, 16 had abdominal involvement, and seven had neurologic signs.20
Diagnosing toxoplasmosis
If T. gondii infection is present, a complete blood count may reveal nonregenerative anemia and leukocytosis. A serum chemistry profile could reveal hypoalbuminemia, hyperglobulinemia, or elevated alanine transaminase, alkaline phosphatase, aspartate transaminase, or creatine kinase activity.9 A fecal examination may show oocysts if the cat is actively shedding. However, serum antibody testing is necessary to confirm the diagnosis.
The cat in this case had a high normal globulin concentration and a lymphocytosis, but it was the high IgM concentrations against T. gondii that confirmed the diagnosis. Since IgM is not detectable nine weeks after infection, high IgM concentrations indicate recent infection.4 A positive IgM titer or a fourfold increase in IgG titers verifies recent infection.
The cat's IgG concentrations were indicative of either exposure or infection. IgG antibodies develop about two weeks after infection; they may remain elevated for years even in healthy animals. About 53% of healthy cats have tested positive for IgG antibodies to Toxoplasma species.7 Thus, high IgG titers do not prove recent or active infection. Conversely, studies have shown that a positive IgM titer was present in only 1.2% of healthy cats.21 After exposure in experimentally infected cats, a peak rise in IgG titers was reached in two to three weeks.9 Ideally, this cat's IgG titer would have been rechecked two to three weeks later to document a rise in convalescent titers. PCR testing to detect the Toxoplasma species DNA in the aqueous humor may also have confirmed the diagnosis.2 However, the clients declined further testing.
Treating toxoplasmosis
Azithromycin was chosen in case this cat had bartonellosis or toxoplasmosis. Systemic drugs used to treat toxoplasmosis are protozoal-static and only influence the tachyzoite phase; thus, they will not kill tissue cysts and will not prevent recurrent infection. Clindamycin may also be used to treat T. gondii infection.22
PROGNOSIS
The prognosis for toxoplasmosis varies depending on the tissues affected and the age at which the cat is infected. Adults that ingest bradyzoites may develop self-limiting diarrhea or be asymptomatic. However, T. gondii infection can be rapidly progressive and fatal in cats that have respiratory and central nervous system signs.
CONCLUSION
Consider T. gondii infection in an outdoor cat with uveitis even if it has no other clinical signs, as uveitis and chorioretinitis may occur without other systemic signs.9
Lyndsey Larson, VMD
Wheat Ridge Animal Hospital
3695 Kipling St.
Wheat Ridge, CO 80033
Todd Hammond, DVM, MS, DACVO
The Veterinary Eye Clinic
7630 W. 39th Ave.
Wheat Ridge, CO 80033
ACKNOWLEDGMENTS
A special thanks to Lori Wise, DVM, MS, DACVIM; Elisa Mazzaferro, DVM, PhD, DACVECC; Brad Graham, DVM, MS, DACVO; and Gary Loeffler.
REFERENCES
1. Glaze MB, Gelatt KN. Feline ophthalmology. In: Gelatt KN, ed. Veterinary ophthalmology. 3rd ed. Philadelphia, Pa: Lippincott Williams and Wilkins, 1999;997-1052.
2. Powell CC, Lappin MR. Causes of feline uveitis. Compend Contin Educ Pract Vet 2001;23(3):128-140.
3. Severin G. Anterior chamber and anterior uvea. Severin's veterinary ophthalmology notes. 3rd ed. Fort Collins, Colo: Veterinary Ophthalmology Notes, 1995;351-378.
4. August JR. Infectious causes of uveitis. In: Consultations in feline internal medicine, volume 5. Philadelphia, Pa: WB Saunders Co, 2006;21-27.
5. Powell CC, Lappin MR. Diagnosis and treatment of feline uveitis. Compend Contin Educ Pract Vet 2001;23(3):258–266.
6. Ketring KL, Zuckerman EE, Hardy WD Jr. Bartonella: a new etiological agent of feline ocular disease. J Am Anim Hosp Assoc 2004;40(1):6-12.
7. Lappin MR. Feline infectious uveitis. J Feline Med Surg 2000;2(3):159-163.
8. English R. Immune responses and the eye. In: Gelatt KN, ed. Veterinary ophthalmology. 3rd ed. Philadelphia, Pa: Lippincott Williams and Wilkins, 1999;239-258.
9. Greene CE. Toxoplasmosis and neosporosis. In: Infectious diseases of the dog and cat. 3rd ed. St Louis, Mo: WB Saunders Co, 2006;754-774.
10. Gelatt K, Gelatt J. Surgical procedures of the anterior chamter and anterior uvea. In: Small animal ophthalmic surgery. Philadelphia, Pa: Butterworth Heinemann, 2003;232-235.
11. Plumb D .Veterinary drug handbook. 5th ed. Boston, Mass: Blackwell Publishing, 2005;116-118.
12. Holmberg BJ, Maggs DJ. The use of corticosteroids to treat ocular inflammation. Vet Clin North Am Small Anim Pract 2004;34(3):693–705.
13. Brooks DE, Ollivier FJ. Matrix metalloproteinase inhibition in corneal ulceration. Vet Clin North Am Small Anim Pract 2004;34(3):611-622.
14. Lappin, MR. General concepts in zoonotic disease control. Vet Clin North Am Small Anim Pract 2005;35(1):1-20.
15. Stiles J. Ocular manifestations of systemic disease. The cat. In: Gelatt KN, ed. Veterinary ophthalmology. 3rd ed. Philadelphia, Pa: Lippincott Williams and Wilkins, 1999;1448-1473.
16. Lappin MR, Marks A, Greene CE, et al. Serologic prevalence of selected infectious diseases in cats with uveitis. J Am Vet Med Assoc 1992;201(7):1005-1009.
17. Klauss G, Constantinescu G. Nonhypotensive autonomic agents in veterinary ophthalmology. Vet Clin North Am Small Anim Pract 2004;34(3):777-800.
18. Powell CC, Lappin MR. Clinical ocular toxoplasmosis in neonatal kittens. Vet Ophthalmol 2001;4(2):87-92.
19. Davidson MG, English RV. Feline ocular toxoplasmosis. Vet Ophthalmol 1998;1:71-80.
20. Dubey JP, Carpenter JL. Histologically confirmed clinical toxoplasmosis in cats: 100 cases (1952-1990). J Am Vet Med Assoc 1993;203(11):1556-1566.
21. Lappin MR, Greene CE, Prestwood AK, et al. Diagnosis of recent Toxoplasma gondii infection in cats by use of an enzyme-linked immunosorbent assay for immunoglobulin M. Am J Vet Res 1989;50(9):1580-1585.
22. Davidson MG. Toxoplasmosis. Vet Clin North Am Small Anim Pract 2000;30(5):1051-1062.