Chronic kidney disease in cats (Proceedings)
Chronic kidney disease and failure is invariably progressive; however, stable disease and a reasonable quality of life can be obtained for some time in most cats.
Chronic kidney disease and failure is invariably progressive; however, stable disease and a reasonable quality of life can be obtained for some time in most cats. A step-wise therapeutic approach that addresses the potential causes and progressive complications of renal failure is suggested. Dietary modification and judicious use of adjunct medications can reduce signs of uremia and prolong survival. Serial monitoring is recommended to best plan management strategies and to assess long-term prognosis. The typical data base includes body weight, CBC or PCV, biochemistry and acid-base measures, urinalysis, indirect blood pressure measurement, and urine culture.
Pathophysiology of Renal Failure
The kidneys perform many metabolic, excretory and regulatory functions in the body. Progression of renal dysfunction is predictable in chronic renal failure, and likely results from increased function and adaptive processes in surviving nephrons, intraglomerular hypertension or systemic hypertension, hyperphosphatemia and renal mineralization, ongoing inflammation, hypokalemia, and proteinuria which may contribute to tubular damage. Common consequences of renal failure (and the mechanisms involved) include:
- Uremic gastroenteritis (Stimulation of chemoreceptor trigger zone, hypergastrinemia, uremic vasculitis)
- Systemic hypertension (glomerular capillary injury, decreased vasodilatory substances, enhanced renin-angiotensin-aldosterone activation)
- Proteinuria
- Hypokalemia and weakness (renal potassium loss, decreased intake)
- Hyperphosphatemia and secondary hyperparathyroidism (decreased GFR, retained phosphorus, decreased activation of vitamin D, decreased calcium absorption)
- Progressive increases in PTH occur to maintain serum calcium concentration
- Metabolic acidosis (retention of acids, failure to resorb bicarbonate, fatigue of ammoniagenesis in nephrons)
- Non-regenerative anemia (decreased erythropoietin production, gastrointestinal loss, shortened RBC lifespan)
- Dehydration and constipation (fluid loss)
Less common consequences of renal failure, usually seen with advanced disease, include bleeding due to platelet dysfunction, uremic encephalopathy, uremic pneumonitis and peripheral neuropathy.
Mechanisms of Progression and Possible Renoprotective Strategies
- Glomerular hypertension: may be minimized by dietary protein reduction, angiotensin converting enzyme (ACE) inhibitors, fish oil supplementation
- Proteinuria: dietary protein reduction, ACE inhibitors, fish oil supplementation
- Systemic hypertension: dietary sodium reduction, calcium channel blocking agents, ACE inhibitors
- Tubulointerstitial injury: fish oil supplementation
- Acidosis:Alkalinizing diet or alkalinizing agents
- Mineral Imbalance and Soft Tissue Mineralization: Dietary phosphorus reduction, calcitriol administration, phosphate-binding agents
Epidemiology and Progression of Chronic Kidney Disease in Cats
General
Chronic kidney disease or failure is common in cats, representing 25 – 50% of cats > 10 years old recorded in the Purdue VM Database in 2000. Regardless of the initial insult or disease process, Chronic Renal Failure is a slowly progressive disorder, beginning with the earliest insult to the kidneys. Some progression may be due to the primary disease process, with natural cycles of progression, remission or exacerbation. Progression is also thought to be due to the long term results of some compensatory mechanisms that occur in remaining nephrons (the "Self perpetuation theory"). The fact that some compensatory mechanisms can lead to deleterious consequences also can be called the "trade off hypothesis". Progression in dogs is fairly linear, whereas clinical research suggests a slower decline in cats, with long periods of stable disease (up to 2 or more years) followed by sudden increases in serum creatinine (Ross et al, 2005). Mechanisms of progression include the effects of glomerular hypertrophy or hypertension in remaining nephrons, deleterious effects of proteinuria and hypokalemia in tubules, ongoing tubulointerstitial inflammation, and damaging effects of the ensuing systemic hypertension, acidosis, and soft tissue mineralization. Current documentation supports systemic hypertension and proteinuria as primary risk factors for progressive renal failure in dogs and cats. Understanding these slowly developing consequences of renal failure provides us the opportunity to intervene with medical strategies designed to blunt the compensatory response or its pathologic results, and can be introduced step-wise at different stages of disease.
Screening for CKD (Early diagnosis)
- Geriatric screening: A minimum data base, including complete urinalysis and thyroid hormone measurements in cats can be recommended in cats older than 7 or 8 years. However, changes in urea and creatinine are considered late indicators of renal disease.
- Monitor high risk cats: geriatric cats, cats with urolithiasis, history of familial disease, history of urinary tract infections
- Chart urine specific gravity: A gradual drop in urine specific gravity may be the earliest laboratory sign of renal disease. Astute owners can monitor urine production or collect samples for periodic assessment
- Urine protein measurements: Repeated proteinuria detected by dipstick or screening tests should be quantitated by urine protein:creatinine determination.
- Systemic blood pressure measurements and fundic examination: Can be conducted as part of geriatric screen. Measurements of greater than 160 mm Hg systolic should be followed up with reassessments. Persistent measures > 180 mm Hg warrant treatment.
- Imaging: Uroliths and changes in renal size or shape can be followed up with more specific testing.
Staging of CKD in Cats
Criteria for staging CKD have been developed by an international group of experts (the International Renal Interest Society, IRIS; see www.iris-kidney.com) in order to help practitioners in the logical and often stepwise diagnosis and management of renal disease. The staging criteria are based on three primary measures, the creatinine measurement, urine protein concentration, and blood pressure (Table 1). Specific criteria and recommendations are expected to be updated annually. Remember that staging criteria should be based on laboratory and clinical findings during relatively stable disease periods, not during a crisis or in the face of dehydration. Additionally, creatinine measures vary widely among laboratories (and among different sizes and breeds in dogs and cats); some caution must be advised in adhering to these strict creatinine ranges.

Table 1. Current IRIS Staging Criteria for Cats with Chronic Kidney Disease
Management
Early Chronic Renal Failure (Stages I and II)
First, any reversible contributors to renal failure are addressed if possible. These include sustained systemic hypertension, chronic infection or inflammation, urolithiasis, and urine outflow obstruction. However, by the time of diagnosis in most cats, the cause of chronic renal failure is usually not identifiable or treatable. In these cases, strategies are employed based on the degree of renal dysfunction. The earliest change developing in renal failure is a loss of urine concentrating ability and subsequent polyuria. Extra attention must be paid to ensuring water and moisture intake. Since most cats that eat well will also drink well and take in moisture from their diet, keeping the cat eating is paramount at any stage of renal failure. Fluid intake can be increased by feeding moist diets, providing plenty of fresh water or a drinking fountain, or adding flavored (low sodium) broths or juices to the food.
Next, renal failure leads to azotemia and retention of nitrogenous wastes and phosphorus. The composition of the diet is designed to benefit the failing kidney by limiting the intake of phosphorus and protein precursors, as well as to provide appropriate nutrients to prevent possible deficiencies or other consequences of renal failure. Most commercial diets formulated for renal failure are characterized as follows: reduced sodium, protein and phosphorus; adequate potassium; alkalinizing; high soluble fiber; manipulation of lipids or supplemental omega-3 fatty acids; high palatability and increased caloric density. "Renal diets" now have proved effective in prolonging survival in clinical trials in dogs and cats.
Although renal failure diets are typically potassium supplemented, total body potassium depletion is common in CRF and may contribute to progressive disease. Early supplementation has been suggested to protect the kidneys, but has not been proven to alter the course of disease. However, potassium supplementation is fairly harmless and could be beneficial. Supplementation should be considered if diet alone does not maintain serum potassium in the mid- to high-normal range.
Angiotensin converting enzyme inhibitors (ACEI) may have multiple beneficial effects on the kidney. By limiting Angiotensin II activity, intrarenal vasoconstriction (especially at the efferent arteriole) and intraglomerular hypertension may be reduced. ACE inhibitors have been useful in proteinuric renal disease and are now recommended in cats with even mild proteinuria Note that normal ranges of urine protein have been narrowed considerably after multiple, large prospective studies in cats. A UPC of > 0.4 is now considered abnormal in cats, with UPC of 0.2-0.4 considered "borderline proteinuria." In Europe, weight gain, appetite, perceived quality of life and survival were greater in benazepril treated cats than in cats treated with placebo, especially in cats with more advanced disease. Severity of proteinuria has been associated with shortened survival in CRF cats in one prospective study (Syme et al, 2003). ACEI were effective in reducting proteinuria in long term study in cats; however the effect on survival was not significant (Gunn-Moore et al, 2003) Whether this drug will become a staple early treatment of CRF remains to be seen. Some caution must be advised, as excessive drop in intraglomerular pressure can lead to an acute drop in GFR and worsening azotemia.
Moderate, Stable Renal Failure (Stage II - III)
If not already in place, the dietary modifications as described above are recommended. Dietary intake may become more of a challenge as the cat's general condition declines. Additionally, increased gastrin retention contributes to gastric acidity and gastritis. H2 blocking antacids, such as ranitidine or famotidine, can be useful in preventing vomiting and maintaining appetite. Omeprazole (a proton-pump inhibitor) can be used for more severe gastric ulceration or for cats that do not respond to other antacids, but is more expensive to maintain longterm. Serum potassium should be monitored and potassium supplementation instituted to maintain the potassium concentration in the mid- to high normal range.
If hyperphosphatemia persists or returns despite dietary modification, phosphate binding agents may be administered to help reduce absorption of phosphorus from intestinal tract. These aluminum or calcium salt agents are best given with food; capsules may be better accepted by cats than liquid preparations. Other adverse effects of phosphate binders are constipation and hypercalcemia (calcium salts). The dose and type of phosphate binder is modified to keep plasma phosphorus in the high normal range without hypercalcemia. Some authors recommend a combination of the two for a total dose of 30 – 180 mg/kg/day. If serum phosphorus can be normalized and serum calcium is normal or low, one also may consider calcitriol (active vitamin D3) administration to help promote calcium absorption and minimize secondary hyperparathyroidism. Calcitriol is reported to improve quality of life and survival in canine CRF patients; benefits are less clear in cats. Careful titration of the dose must be used to avoid hypercalcemia. For established renal hyperparathyroidism, 1 – 3 ng/kg/day is given while serum calcium and PTH are monitored for effect (weekly during initial titration).
Anti-hypertensive therapy is indicated if systolic blood pressure is consistently greater than 160-180 mm Hg or if ocular signs are evident. Occasionally retinal vascular change, hemorrhage or detachment are seen in what appear to be normotensive or mildly affected CRF cats. Note that there is great variability in measurements based on technique and personnel used. Dopplers have been the preferred method, but may underestimate blood pressure. After sodium restriction, calcium-channel blocking agents (amlodipine) have been the most effective antihypertensive in cats and have become the first choice drug in cats. Angiotensin-converting-enzyme inhibitors may be added to the regimen for refractory hypertension and may have beneficial effects on renal function and proteinuria in cats (see above). Anti-hypertensive agents can reduce renal blood flow and result in small increases in azotemia; careful monitoring (2 – 4 weeks initially, then at least every 3 months) is warranted.
Progressive acidosis (TCO2 or bicarbonate < 15 mmol/L) may require alkalinization treatment. Potassium citrate is a useful potassium supplement and potential alkalinizing agent, although its effectiveness in creating alkalinization is unknown. Other alkalinizing agents are sodium salts (e.g. sodium bicarbonate), difficult to formulate and administer, and probably should be avoided.
Advanced or Rapidly Progressive Renal Failure (Stage IV)
For cats diagnosed with advanced renal failure or those that progress during treatment, some additional treatment methods may be useful in prolonging a reasonable quality of life. The timing of initiation of any of these strategies will vary with individual cases.
Fluid support becomes more important in advanced cases. Often, short-term diuresis in the hospital will be required for crises. During these times, it is important to frequently assess hydration and to calculate fluid needs based on maintainance and percent dehydration. A supplement of 10 – 20 mls/kg/day for polyuric losses can initially be added to this calculation and adjusted based on the cat's response, rather than using an arbitrary multiple of maintenance needs (e.g. 2x maintenance or 3x maintainence). Fluid overload and sodium overload are possible in cats that are oliguric or not carefully monitored. Longer term fluid supplementation may be accomplished by subcutaneous fluid administration or feeding tube placement. Many cats tolerate subcutaneous fluids well, even in earlier phases of disease. Typical treatments are Lactated Ringer's solution or lower sodium fluid at 75-150 mls/day (roughly 30 mls/kg) for advanced renal failure. Sometimes this fluid supplementation can be tapered to every other day or several times weekly to prevent crises.
Another significant challenge for more advanced cases is maintaining appetite and caloric intake. First, treatments for uremic gastritis should be instituted if not already given. Sucralfate may be added for presumed active gastric ulceration. Topical treatments such as oral gels or tea rinses may be necessary for severe stomatitis. Metoclopramide or other anti-emetics may be needed to minimize vomiting. Low doses (0.2 mg/kg q 8 hrs) are recommended for cats with renal failure. Gastrostomy tube placement and feeding may be useful for cats with poor appetites; food, fluids and medications can be given via a less stressful route.
Progressive anemia also contributes to poor attitude, poor appetite and poor body condition. Anemia can be due to decreased erythropoeisis, blood loss or malnutrition. Treatments include those designed to 1) minimize gastrointestinal bleeding; 2) supplement iron if needed for blood loss anemia, or if EPO is used; 3) provide recombinant erythropoietin administration if severe (darbopoietin is a good alternative and may be less antigenic). EPO should be administered using a low dosage and frequency with the target of a low normal PCV (20-25%). Adverse effects include systemic hypertension, exacerbation of iron deficiency, cross-reacting antibody production (based on cumulative exposure) leading to refractory anemia. Anabolic steroids (particularly stanozolol) are toxic in cats and are not recommended in the management of renal failure.

Table 2. Summary of Chronic Kidney Disease Treatment by Stages in Cats
In certain cats with chronic renal disease, renal transplantation may be an option for long term improvement in renal function. Select veterinary centers perform the procedure, with survival times of 1 – 4 years. Rejection of the transplanted kidney, hypertensive crises, and infection are possible complications. Ideal candidates are young to middle age cats with no infectious disease, cardiac disease or history of urolithiasis. Owners must be willing to travel to the transplant center, accept cost of treatment, adopt the donor cat, and carefully monitor and continue immunosuppressive treatment indefinitely.
Chronic hemodialysis has also been employed for select dogs with chronic disease and may ultimately be a useful adjunct treatment for cats with advanced disease. Availability and expense of treatments remains the key limiting factor for dialytic therapy in animals.
Monitoring and Prognosis
Body weight and condition, minimum data base results, systemic blood pressure, and urine culture should be recorded for most rechecks. Cats should be monitored based on stage, condition and changes in the management plan. Early kidney disease cats can be checked every 3 – 6 months, while late stage cats are rechecked every 3 – 4 weeks. The effects of new treatment strategies should be monitored as indicated above, usually every 2 – 4 weeks. Well managed cats can exhibit stable disease for several years.
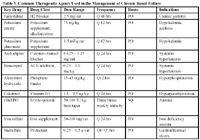
Table 3. Common Therapeutic Agents Used in the Management of Chronic Renal Failure
References
Ross SJ et al (2005). Clinical evaluation of dietary modification for treatment of spontaneous chronic renal failure in cats. Proc ACVIM Annual Forum, Baltimore.
Syme H, Elliott J. (2006) Proteinuria. In August J, ed. Consultations in feline Internal Medicine, 5th ed. Elsevier.
Polzin, et al. (2005) Chronic renal failure. In Ettinger SJ, Feldman EC, eds: Textbook of Veterinary Internal edicine, 6th ed., Elsevier.
Elliot J et al. (2008). Staging Chronic Kidney Disease. In Bonagura and Twedt, eds. Current Veterinary Therapy XIV; Small Animal Practice, forthcoming.