Critical care for small exotic patients (Proceedings)
Although the principles of critical care are universal in all species, veterinarians face a unique set of challenges when dealing with small mammals.
Although the principles of critical care are universal in all species, veterinarians face a unique set of challenges when dealing with small mammals. In true emergencies, time is critical. The nearest veterinarian, who may be unfamiliar with that species, must render care. However, the basic guidelines for critical care can be modified and adapted to address any species. Once stable, general medical principles can be applied to any small exotic species – as long as the veterinarian is aware of the unique aspects of these species. Most needs can be met by adapting equipment found in most veterinary practices.
General Concerns When Caring for Small Patients
Small patients have a much more rapid metabolism than the more familiar domestic pets. This can lead to very rapid use of glucose reserves, and more rapid disturbances in fluid and electrolyte parameters. Perhaps the most important consequence is the much greater susceptibility to hypothermia in most species. (*Exception – chinchilla, hedgehog) Smaller patients are often more difficult to properly restrain without injury to the patient (or veterinarian), and many have a unique predisposition to stress that can cause catecholamine release, cardiac arrhythmia, and even death due to handling and treatment alone. In addition, drug doses are often minuscule, sometimes leaving a negligible difference between dosing and overdosing.
Anesthesia poses a far greater risk to these small patients unless proper precautions are taken. Many species are extremely difficult to intubate, and catecholamine surges can cause cardiac arrhythmias and respiratory depression. These dangers are compounded by a lack of appropriately sized anesthetic monitoring equipment.
Vascular access sites are limited, yet effective catheter placement and fluid therapy is essential in most patients. In addition, because of the very small patient size, even small volumes of blood loss can leave a patient critically hypovolemic.
Therapy for Small Patients
Therapy for small exotic patients should proceed as in any pet. The basic principles of care are universal – provide fluids, heat as necessary, and oxygen. Once these basic needs have been achieved, then the practitioner can address wound care, nutritional support, and appropriate antimicrobial therapies. Always perform a complete physical examination – do not be intimidated by the size or species of the patient. However, it may be preferable to perform the exam in stages, to avoid excessive restraint or distress to the patient. Utilize the systematic approach that is most familiar to you – the approach you use in assessing your canine and feline patients. The most important factor is consistency, which decreases the potential for missing problems.
Emergency Care
When rendering care to the patient presented as an emergency, be sure to assess the entire patient. In the case of an emergency, rapid and limited assessment is appropriate. However, do not assume that the obvious injury is the most severe. For example, a patient appearing weak or even neurologic may have a severe enough anemia to be causing all the other signs; conversely, a patient with a hemorrhaging injury may also have severe head trauma that can be equally life threatening. Always treat the most critical conditions first; stabilize the patient, THEN provide proper wound and supportive care.
Initial Emergency Assessment
The principles of life support and emergency care are universal amongst species. (These principles will be demonstrated below, incorporated with general care techniques.) Follow suggested protocols to assess airway, breathing, and cardiovascular support (ABC's of triage). Control hemorrhage, provide heat, and evaluate for wounds. Assess thoracic and abdominal cavities for penetrating injuries, ruptured organs, masses, or fluid or air accumulations. Postpone care of fractures or non-life-threatening wounds until the patient has been stabilized.
General Care Techniques
Fluid Therapy and Vascular Access
Fluids may be administered through several routes; the preferred route should be determined by patient status.
Subcutaneous
Fluids can be used in stable patients with minimal or no dehydration. Depending on patient size, a 20-22-gauge needle is usually appropriate. Small volumes of fluids can be administered via syringe and butterfly needle for more accurate administration – also provides greater ease of delivery in an active patient.
Ideal Site
Dorsal shoulder/cervical region.
Intravenous Fluids
Small catheters are available – 22, 24, or 26-gauge are available. Most common sites are cephalic or lateral saphenous, which can be accessed in almost any species. Jugular veins can also be used, although cutdown may be required.
Ideal Sites /Rates/Precautions
Ferret
Cephalic, lateral saphenous, (jugular – cutdown; ferrets depressed with neck wrap)
Maintenance: 70-90ml/kg/day
Thick skin; penetrate skin with needle prior to catheter insertion.
Rabbit
Cephalic, lateral saphenous.
(Lateral ear vein can be used in emergency; difficult to maintain and predisposed to hematoma and potential slough). Jugular vein can be used; may cause respiratory discomfort and compromise. Not recommended for fluid administration, but safe for blood collection.
Maintenance:100 ml/kg/day
Thin skin, easily torn, unless intact.
Often chew IV lines
Rodents, Sugar Gliders
Lateral or medial saphenous Maintenance: 50-60 ml/kg/day
Chinchilla, Guinea Pig, Hedgehog, Prairie Dog
Cephalic, lateral or medial saphenous.
Maintenance: 50-60 ml/kg/day
Relatively thin skin. Easy to injure legs of chinchillas with restraint.
Intraosseous
Sometimes the most rapid and effective route for fluid administration in small patients. In larger patients, 20 or 22-gauge spinal needles or bone marrow needles can be used. However, standard hypodermic needles can easily be used. This allows for rapid and effective delivery of fluids and drugs, and can be left in place for continuous or subsequent dosing. Sterile prep should precede intraosseous catheter placement. 22, 23, or 25-gauge needle works well as an intraosseous catheter. Often, the needle is easily placed but may be occluded by a bone core in the needle. If this occurs, remove the initial needle and replace with a second needle of equal size. The entry site in the bone is easily relocated by gently sliding the needle tip along the bone at the approximate entry site.
Ideal Sites
Femur (through trochanteric fossa); Tibia (through tibial crest).
Intraperitoneal
Lower right quadrant is preferred to avoid most important organs. Hold patient in dorsal recumbency so organs fall towards spine; aspirate first to assure that there is not penetration of an organ.
Choice of Fluids
Crystalloids
LRS, Normasol, plasma-lyte, Normasol: Rapid volume replacement, dehydration, ongoing losses.
Colloids
Hetastarch – can be used to improve oncotic pressure, replace/correct hypoproteinemia, or hypovolemia, particularly in crisis situation. Also beneficial in renal disease, any disease causing decreased perfusion. May aid in acute hemorrhagic crisis in addition to transfusion.
10-15 ml/kg over 20-40 minutes, up to QID, OR
10-15 ml/kg bolus over 20-40 minutes followed by 1-2 ml/kg/hr continuous rate infusion.
Recommended maximum dose 20 ml/kg/24 hours. (*Author's note: I have exceeded this dose with no side effects noted.) See section below on CPCR for modification during resuscitation.
Blood – can be administered for RBCs, platelets & clotting factors, and hypoproteinemia. Can be obtained from a donor; often a cage mate, sibling, or offspring. Some shelters (ferret, rabbit) or owners are willing to provide donors; purchase of a donor pet from a pet shop is another option, although health cannot be guaranteed. (*Author's note: Please advocate that the owners must be responsible for care/adoption of the donor pet.) Fresh blood is collected from a donor into ACD or appropriate anticoagulant (Heparin can also be used). (Either ACD or heparin is used at a 1:10 to 2:10 ratio of anticoagulant:blood) The transfusion is given slowly over 20-30 minutes, monitoring for transfusion reactions. It is best to administer by hand, rather than infusion pump, as any excess pressure can lyse cells. There is only one blood type in ferrets, and transfusion reactions are unlikely. Information is limited in other species. Minor cross-match can be performed in house.
Maximum to collect from donor: 1% of body weight in kilograms. (7-10% of body weight is blood volume; 7-10% of that can safely be collected from healthy patients. High end is recommended due to small volume. ) Donors should be anesthetized for collection (isoflurane recommended). Collection usually from cranial vena cava, jugular, central auricular artery (rabbits) or cardiac (not recommended in an already owned family pet).
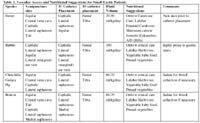
Table 1. Vascular Access and Nutritional Suggestions for Small Exotic Patients
Fluids should be warmed when administered to any critical patient. Hypothermia is often overlooked as a contributing factor to shock in critical patients, particularly small patients. However, with the small volumes administered, heat is rapidly lost from a warmed bag as the fluid travels through the tubing. One simple solution to administer warmed fluids and provide patient warmth is the use of warmed rice bags. Uncooked rice is placed into a nylon stocking, which is knotted to keep the rice in. These bags are heated in the microwave for 1.5-2 minutes. Fluid lines can be coiled underneath these warmed bags, warming fluids as they enter the patient. These bags offer an advantage over warm water bottles or gloves, as they will never draw heat away from the patient, as water will once it cools.

Table 2. Anesthesia Protocols by Species
Respiratory Support and Oxygen Administration
Airway control is easily established in some species, such as ferrets; but is nearly impossible in others (chinchillas, rodents). Intubation is achieved with a 2.5 to 3.5 mm cuffed endotracheal tube in large species, and positive pressure ventilation can be provided using oxygen or an ambu-bag. For smaller species, Cole tubes may be useful (stepwise increase in tube lumen), as may small red rubber tubes cut to appropriate lengths (with 1-2 additional air holes cut in distal aspect). If excessive tracheal secretions are present, suction can be provided by using a red-rubber tube either alone or through the lumen of an endotracheal tube. Endotracheal tube cuffs should not be inflated in birds or reptiles, as they have complete tracheal rings and inflation may cause severe tracheal necrosis.

Table 3. Antimicrobials Commonly Used
In an emergency situation in which airway control is essential, oftentimes it is more practical to perform a tracheotomy than to spend several minutes attempting intubation. Quickly clip and prep the skin; the skin incision is made lengthwise, trachea exposed, and the incision into the trachea is made horizontally in between rings. Ideally the incision is ¼ the tracheal lumen or less. Once the endotracheal tube is in place, ventilation proceeds as in any other species. Rapid ventilation is recommended due to the increased resting respiration rates and rapid oxygen depletion in small patients. (approx. 1 breath per second)
In patients that have a patent airway and require supplemental oxygen, several routes are available that can be used in most practices. Oxygen cages are the least desirable for long-term oxygen therapy but can easily be created using in induction chamber, or a patient carrier covered by plastic, leaving a small opening for expired CO2. A facemask can also be used to deliver oxygen for a short period, and can be created from a syringe case if commercial facemasks are not available. Nasal oxygen can be administered through a small Teflon catheter or red rubber tube inserted into the ventral nasal meatus and advanced to the level of the medial canthus of the eye. Oxygen collars can also be made from radiographic film and covered with clear plastic, leaving an opening for expired gas. However, this may be impractical in species which do not tolerate Elizabethan collars for prolonged periods.
Cardiopulmonary Cerebral Resuscitation (CPCR)
CPCR is often necessary in critical patients. For these small patients, current trends lean towards small-volume fluid resuscitation with frequent reassessment rather than large boluses of fluids. For the shocky debilitated patient, Hypertonic saline is administered (3-5 ml/kg) over 5 minutes, followed by Hetastarch (3-5 ml/kg over 5 min). This combination prior to crystalloid administration enables fluids to stay within the vascular space. Blood pressure and body temperature should be monitored closely; if body temperature remains <98 (exception: chinchillas, hedgehogs, sugar gliders, whose normal body temperature can be lower), then begin administration of small boluses of 3-4 ml/hr of crystalloids while providing heat support. Once body temperature reaches 98, small boluses of crystalloid fluids (LRS, Plasma-lyte) are administered (15-20ml/kg) along with Hetastarch (3-5ml/kg) over 15 minutes, and the patient is reassessed every 15 minutes. The crystalloids and Hetastarch can be combined in the same syringe or bag. This process is repeated every 15-20 minutes until temperature normalizes and blood pressure is over 90 mmHg. When body temperature is below 98, in some animals, they may develop pulmonary edema due to the accumulation and inappropriate distribution of fluids within the body, leading to cardiac overload.
Supportive Care
Antimicrobial Therapy
Antimicrobial choice may be more limited when treating small exotic patients. The choice of drug should be based on bacterial culture and sensitivity whenever possible. The drug must also be an appropriate drug for the species of patient, as many patients may have severe complications from inappropriate use of inappropriate antimicrobials. Of equal importance is providing an appropriate dose and route of administration. Most pill formations are dosages that are far in excess of the required dose for a small patient, and cutting a pill into eighths or tenths leads to extreme inaccuracy in dosing. In addition, it is extremely difficult to pill most small exotic species. When oral medications are indicated, commercially available or compounded suspensions are preferable. In-house compounding is not recommended, as many drugs are unstable unless a suspending agent is used. Drugs can also be administered by the intramuscular or subcutaneous routes. Insulin or tuberculin syringes provide more accurate dosing than larger syringes.
Nutritional Support
The rapid metabolism of most small exotic patients leads to rapid depletion of glucose reserves. Nutritional support is essential in these patients, some requiring feeding as frequently as four times daily. Fasting should be avoided in most patients.
Oral or enteral routes should be used whenever possible to maintain intestinal absorptive capability. Some species can easily be encouraged to eat or force fed (ferrets, rabbits, rodents); while others may be easily gavage fed (some small rodents, ferrets). Suggested foods for force-feeding are listed in Table 1. A pharyngostomy or esophagostomy tube can be placed, although many patients will attempt to remove these. If indicated or desired by owners, total parenteral nutrition can be administered through 22-ga or larger intravenous catheter in small mammals, and is typically far less expensive than in dogs or cats, simply because a 1-L bag can be used for 5-7 days.
Newsletter
From exam room tips to practice management insights, get trusted veterinary news delivered straight to your inbox—subscribe to dvm360.