Current guidelines for cardiopulmonary cerebral resuscitation (Proceedings)
Cardiopulmonary cerebral resuscitation (CPCR) refers to the re-establishment of circulation and preservation of neurologic function following an arrest.
Cardiopulmonary cerebral resuscitation (CPCR) refers to the re-establishment of circulation and preservation of neurologic function following an arrest.1 Since its inception in the late 1800's, CPCR has saved the lives of countless human and veterinary patients. However, low overall survival rates following CPCR indicate that there is still much room for improvement in these practices. This session reviews current practices and updates on CPCR in the veterinary patient.
Basic Life Support
Basic life support refers to the process of establishing an airway, initiating positive pressure ventilation, and performing chest compressions. Because cardiopulmonary arrest (CPA) in veterinary patients is frequently initiated by respiratory arrest, an ABC approach is generally taken as described below.
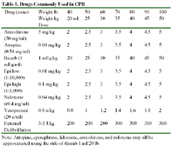
Table 1. Drugs Commonly Used in CPR
Airway
Orotracheal intubation is easily achieved in dogs, as the larynx can be directly visualized by retracting the tongue. The head and neck should be gently extended and a laryngoscope may be used to improve visualization of the larynx. In cases where hemorrhage, saliva, or gastric contents interfere with visualization, suction may be helpful. Alternately, the glottis may be palpated with one finger used to guide tube placement. Once tube placement is verified, the tube should be secured by tying to the nose or around the back of the head. The cuff should be inflated, and assisted ventilation provided. If chest wall excursion is not seen, lung sounds are absent, or abdominal distension is noted, tube placement should be reconfirmed by direct visualization and the cuff should be reinflated. Improper tube placement and tube dislodgement are common causes of CPCR failure.
Breathing
Once an endotracheal tube is in place, breathing is initiated at a rate of 10-20 breaths per minute with 100% oxygen. An ambu bag with attached oxygen line is ideal for this purpose. If only one person is available to perform CPR, 2 breaths should be given for each 15-30 chest compressions. If several trained personnel are available, then breaths may be delivered independent of compressions. Chest wall excursion should be seen with each delivered breath. Airway pressures ideally should not exceed 20-30 cm H2O. High airway pressures or inadequate chest wall excursion should prompt a search for pleural space disease, tube malposition, or tube occlusion.
Circulation
Chest compressions are initiated at a rate of 100-120 per minute, compressing the circumference of the chest by approximately 30%. The patient should be in lateral recumbency during compressions. In smaller dogs, where the cardiac pump theory is believed to predominate, hands should be placed over the ventral third of the chest just behind the point of the elbow, corresponding to a position directly over the heart. In larger dogs, the thoracic pump theory is believed to be most important in generating blood flow, and hands should therefore be placed over the widest part of the thorax to create a maximal rise in intrathoracic pressure.
A number of alternative techniques have been investigated that may help to augment blood flow during CPCR. Those that are directly applicable in veterinary patients include circumferential chest compression and interposed abdominal compressions. Circumferential chest compression is most commonly performed in cats and small dogs by encircling the chest with both hands to maximize the rise in intrathoracic pressure during chest compression. In larger animals, interposed abdominal compression may be implemented by having an additional person perform abdominal compressions during the relaxation phase between chest compressions. Interposed abdominal compressions increase venous return to the heart, leading to greater stroke volumes and cardiac output, and have been associated with increased survival to discharge in human patients.
Advanced Life Support
Advanced life support consists of drug administration, determination of cardiac electrical activity, and application of electrical defibrillation if indicated. These techniques build upon basic life support to increase the likelihood of successful resuscitation.
Drugs
Establishing vascular access is one of the first priorities during advanced life support. While central lines are preferable for rapid distribution of drugs, peripheral catheters are acceptable, and drug delivery may be facilitated by following drug administration with a 10-20 ml IV fluid "chaser". If vascular access is not immediately obtained, surgical cutdown or intraosseous techniques should be considered. The intratracheal route may also be used initially to deliver drugs. Epinephrine, atropine, vasopressin, lidocaine, and naloxone may all be given in this way by administering twice the normal dose of the drug and administering several large breaths to disperse the drug.
Drugs administered during CPCR include intravenous fluids, narcotic reversal agents, vasopressors, vagolytics, antiarrhythmics, and potentially sodium bicarbonate. Shock doses of intravenous fluids should be provided in cases where hypovolemia is believed to have played a role in the arrest. Moderate fluid rates should be used in euvolemic patients or patients with underlying heart disease, as rapid administration in these cases may excessively elevate right atrial pressure and actually decrease myocardial and cerebral perfusion pressure.
Patients who have received narcotic pain relievers or other sedative/anesthetic drugs prior to arrest should immediately be given the reversal agent for that drug. Naloxone may be used to reverse most narcotics at a dose that is isovolumetric to the dose of the original narcotic, or at 0.02-0.04 mg/kg IV if the original dose is unknown. Flumazenil (0.02 mg/kg IV) may be used to reverse benzodiazepines, and yohimbine (0.1 mg/kg) or medetomidine (0.2 mg/kg or isovolumetric) may be used to reverse xylazine and medetomidine respectively. Any anesthetic gases, if still in use, should be discontinued and the anesthetic circuit flushed with fresh oxygen.
Vasopressors are commonly used during CPCR to increase blood pressure and redistribute blood flow to vital organs like the brain and heart. Epinephrine continues to be the vasopressor of choice during CPCR in veterinary patients, though its use is largely extrapolated from clinical studies in human patients. Both low dose and high dose epinephrine protocols are described in human medicine. While high dose epinephrine has been associated with increases in early return of spontaneous circulation, no long-term benefits have been identified. High dose epinephrine has additionally been associated with increased myocardial oxygen demand and worse neurologic outcomes.2 For these reasons, it is recommended that low dose epinephrine initially be administered every 3-5 minutes during CPCR, switching to the high dose only if there is a lack of response to the lower doses. Epinephrine dosing may be rapidly calculated according to the following rule of thumb: 1 ml per 20 lb of the 1:10,000 formulation for low dose, or of the 1:1,000 formulation for high dose.
Vasopressin is another potent vasoconstrictor that is increasingly used in resuscitation of human patients. Unlike epinephrine, it does not increase myocardial workload, and its effect is not blunted by acidosis. Although clinical data in veterinary patients is currently lacking, animal models and human clinical trials suggest that vasopressin may be as effective as, or superior to, epinephrine.3 Vasopressin (0.8 units/kg IV) may therefore be considered as an alternative to epinephrine in dogs.4
Atropine is another drug frequently administered during CPCR to reverse parasympathetic contribution to the arrest or to treat sinus bradycardia. Atropine is administered at a dose of approximately 1 ml per 20 lb (0.04 mg/kg) for asystole or pulseless electrical activity. When treating sinus bradycardia, only half this dose is needed.
Sodium bicarbonate use in CPCR is controversial, as it has been associated with numerous adverse effects including hypernatremia, paradoxical CNS acidosis, and decreased resuscitation rates in people. However, its use should still be considered during long duration (>10 minutes) arrests, as control of acidosis may improve response to catecholamines as well as post-arrest neurologic outcomes. Bicarbonate is typically given only after 10 minutes of CPCR at a dose of 1 mEq/kg and is repeated every 5 minutes thereafter.
Electrical Activity
ECG leads should be attached as soon as feasible to assess electrical activity. Connecting the leads to the skin of the lower forelimbs and hindlimbs will help to minimize motion artifact associated with resuscitation efforts. Four rhythms are commonly seen during cardiopulmonary arrest in dogs. Asystole and pulseless electrical activity are the initial arrest rhythms most commonly seen in dogs, followed by ventricular fibrillation and sinus bradycardia.5,6 Accurate ECG diagnosis is vital to a successful code. The presence of sinus bradycardia or suspicion of a vagal arrest should prompt administration of atropine. Asystole should be confirmed in more than one lead, to rule out the possibility of artifact related to poor contact. While some dogs in asystole will convert directly to sinus rhythm following resuscitation, many develop ventricular fibrillation and require electrical shock for conversion. Once ventricular fibrillation is identified, electrical defibrillation should immediately be administered, temporarily bypassing all other resuscitation measures. The greater the time that a dog spends in fibrillation, the lower the likelihood of successful conversion.
Defibrillation
Early application of electrical shock is the only effective method for converting VF to sinus rhythm. VF is a form of disorganized electrical activity with various portions of the heart muscle firing at different times. Electrical shock essentially "resets" the cardiac cells so that organized activity can resume. Practically speaking, applied current must pass through at least 30% of cardiac myocytes to effectively convert VF.
To accomplish defibrillation, the dog is flipped into dorsal recumbency immediately preceding defibrillation and handheld paddles are placed on either side of the chest directly over the heart. Ample conducting gel should be applied to the paddles to ensure good contact and prevent dispersion of current. The chest should be compressed between the paddles, minimizing impedance by narrowing the distance between paddles. Three shocks are typically delivered in sequence, pausing in between each shock only long enough to assess rhythm and recharge the paddles. Electrical shock is discontinued once the rhythm converts from VF. The energy for the first two shocks should be set at 3-5 J/kg, increasing by 50% for the third shock, if needed. If defibrillation is not successful, CPCR is resumed for 60-90 seconds and the next series of shocks is given at 1.5 times the initial energy level.
For shock-refractory VF, a search should be undertaken to identify problems such as improper paddle position, inadequate contact, insufficient conduction gel, or the presence of pleural space disease that may increase impedance. Drug-shock techniques may then be considered, administering epinephrine or amiodarone (5 mg/kg IV) prior to shock to lower defibrillation threshold. Lidocaine was previously used for this purpose as well, but has been reclassified as a therapy of indeterminate benefit in the most recent ACLS guidelines.7
Open Chest CPCR
There are a number of absolute indications for open chest CPCR. These include cardiac arrest caused by or associated with pleural space disease (pneumothorax, pleural effusion, diaphragmatic hernia), pericardial effusion, or penetrating injury resulted in cardiac arrest. However, debate exists in veterinary medicine as to other indications for performing open chest CPCR. Some advocate open chest CPCR immediately in large breed dogs because of the limited success of restoring adequate circulation with external compressions while others prefer to perform external CPCR for 5 minutes and then open the chest if there is little or no evidence of effective circulation. Open chest CPCR has the advantage of allowing the clinician to directly compress the heart and improve stroke volume. In addition, opening the chest makes assessment of ventricular filling feasible aiding in the decision of volume delivery.
When opening the chest, it is critical to auscult the chest just prior to the incision to rule out ECG dysfunction as the cause of asystole! The left chest should crudely clipped of hair at the left 5th -6th intercostals space and a chlorhexidine based antiseptic solution should be briskly applied. An incision should be made through the skin and subcutaneous tissues from just below the spinal musculature to the level of the costochondral junction. Between positive pressure breaths, mayo scissors should be used to poke through the intercostal musculature and the pleura and the chest is opened by sliding the mayo scissors dorsally and ventrally along the cranial border of the rib (to avoid the neurovascular bundle). The pericardium is opened at the pericardio-diaphragmatic ligament and the heart is compressed from the apex to the base. In large dogs, the heart can be compressed against the opposite chest wall.
In the event of return of spontaneous circulation, antibiotics should be instituted immediately, the chest should be lavaged with copious amounts of warm saline, and should be closed using sterile technique over a chest tube.
ICU Care
Following a successful code, a search for underlying causes or complications should be performed and any problems corrected. Blood gases, hematocrit and total solids, blood pressure, and oxygen saturation are carefully monitored and optimized during this time. This tends to be the most challenging phase of arrest management, as complications and recurrence of CPA are common. Neurologic recovery is promoted by maintaining arterial blood pressure and oxygen saturation. Because elevation in carbon dioxide levels leads to cerebral vasodilation and consequently increased intracranial pressure, hypercarbia should be prevented by employing mechanical ventilation if needed. Once cardiovascularly stable, mannitol (0.25-0.5 g/kg IV over 20 minutes) may also be indicated to treat cerebral edema and resultant elevations in intracranial pressure. Corticosteroids are associated with potentially deleterious hyperglycemia in post-arrest patients, and current protocols do not support their use.7
Prognosis
Recurrence of CPA in the post-arrest period is common, occurring in up to 70% of successfully resuscitated dogs. Intensive care and monitoring during this time is therefore essential. Survival to discharge following cardiopulmonary arrest has been reported in 4-11% of cases.5,6,8 Transient blindness, seizures, circling, ataxia, and decreased level of consciousness are common for some period of time following CPA, but the majority of survivors have a good prognosis for functional recovery.6
References
Otto CM. Cardiopulmonary cerebral resuscitation and ACLS guidelines. In Proceedings of the 8th International Veterinary Emergency and Critical Care Symposium. San Antonio, TX. September 2002. p. 478-481.
Gueugniaud PY, Mols P, Goldstein P. et al. A comparison of repeated high doses and repeated standard doses of epinephrine for cardiac arrest outside the hospital. N Engl J Med 1998;339:1595-1601.
Wenzel V, Krismer AC, Lindner KH, et al. Comparison of vasopressin and epinephrine for out-of-hospital cardiopulmonary resuscitation. N Engl J Med 2004;350:105-113.
Schmittinger CA, Astner S, Astner L, et al. Cardiopulmonary resuscitation with vasopressin in a dog. Vet Anaest Analg 2005;32:112-114.
Wingfield WA, Van Pelt DR. Respiratory and cardiopulmonary arrest in dogs and cats:265 cases (1986-1991). J Am Vet Med Assoc 1992;200:1993-1996
Waldrop JE, Rozanski EA, Swanke ED, et al. Causes of cardiopulmonary arrest, resuscitation management, and functional outcome in dogs and cats surviving cardiopulmonary arrest. J Vet Emerg Crit Care 2004;14:22-29.
American Heart Association. Guidelines 2000 for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation 2000;102(suppl.):I1-I384.
Kass PH, Haskins SC. Survival following cardiopulmonary resuscitation in dogs and cats. J Vet Emerg Crit Care 1992;2:57-65.