Cushing's controversies (Proceedings)
None of the tests for hyperadrenocorticism (HAC) in dogs are perfect.
WHICH SCREENING TEST IS BEST?
None of the tests for hyperadrenocorticism (HAC) in dogs are perfect. It could be reasonably argued, in fact, that the most important clinical tests for HAC in the dog are a thorough clinical history and a good physical examination. If there is not a reasonable clinical index of suspicion, none of the endocrine tests are valid.
In general, it makes sense to use a test designed to suppress hormone secretion in order to diagnose a disease of hormone excess. Likewise, diagnosis of a disease in which hormone secretion is lacking might be best diagnosed by attempting to stimulate secretion. Both types of tests are used commonly in diagnosis of HAC in dogs. The Adrenocorticotropin (ACTH) stimulation test is popular because of the short amount of time it takes to perform the test. The Low-dose dexamethasone suppression test (LDDST) takes at least 8 hours to perform, but it costs considerably less than the ACTH stimulation test because of the relative expense of ACTH compared to dexamethasone. But is one test better than the other?
The LDDST is a highly sensitive test; when various reports are combined, the sensitivity of the test is approximately 95%. The specificity, however, appears to be low, with estimates ranging from approximately 45% to 75%. This means that the chance of a false positive is quite high. The sensitivity of the ACTH stimulation test is generally considered to be lower, approximately 80%. The sensitivity for pituitary-dependent HAC is considerably higher. Few studies have compared the two tests. One study of 40 necropsy-proven cases of canine HAC found that the ACTH have sensitivity and specificity of 95% and 91%, respectively, while the LDDST was 96% sensitive and 70% specific.1 Another study of 59 dogs with non-adrenal illness shoed that the LDDST gave positive results in 56% of dogs, while the ACTH stimulation test gave positive test results in 14% of dogs.2 These studies would seem to favor the ACTH stimulation test over the LDDST for diagnostic accuracy. The LDDST is, however, not without its unique value. Of the two commonly used screening tests, only the LDDST can confirm pituitary-dependent HAC if the 4-hour post-dexamethasone serum cortisol concentration is less than 1 mcg/dl and it escapes from suppression at 8 hours. In addition, the chance of a false negative test in a dog with an adrenal tumor approaches 0% for the LDDST, while the sensitivity of the ACTH stimulation test for adrenal tumors is only around 60%.
Based on evidence, there is no good reason to choose one test over the other. I prefer the LDDST because of its high negative predictive value. A positive test result, however, should never be taken as strong evidence of HAC in an animal with clinical signs that are not strongly suggestive of the disease.
DOES ATPICAL CUSHING'S SYNDROME EXIST?
Atypical HAC is defined as the clinical syndrome of HAC in which results of LDDST, ACTH stimulation test, or both, are normal, and there is an increase in the serum concentration of 17-alpha-hydroxyprogesterone (17OHP). Recent studies have shown increased ACTH-stimulated 17OHP in dogs suspected of having atypical HAC, and this has led to the theory that HAC can be caused primarily by increased circulating concentrations of steroidogenesis precursors.3,4 This theory is difficult to prove given the lack of specificity and sensitivity of more conventional screening tests. It should not be surprising that cortisol precursors would be abnormal in a dog with HAC, but are these precursors clinically relevant? Figure 1 shows the biochemical pathway for steroidogenesis in the adrenal cortex. If 17OHP was abnormally high, what would happen to the production of cortisol? If further metabolism to 11-deoxycortisol was blocked, resulting in high 17OHP, serum cortisol in dogs with atypical Cushing's syndrome would be low. If 17OHP production was increased due to increased enzymatic activity at an upstream point, serum concentrations of cortisol would likely also be increased. In fact, 17OHP concentrations are typically increased in dogs with conventionally diagnosed HAC.5 . In most cases of atypical HAC, however, results of ACTH stimulation testing and LDDST are equivocal. This is no different from many cases of HAC.
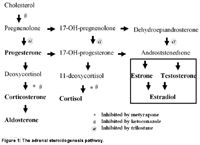
Figure 1: The adrenal steroidogenesis pathway.
When making a diagnosis of HAC based on results of 17OHP testing, extra care should be taken to ensure that a false diagnosis is not made. In one study, serum concentrations of 17OHP were high in more than 30% of dogs with nonadrenal neoplasia and that were suspected of having HAC but later confirmed negative for the disease.6
IS TRILOSTANE THE ANSWER?
Trilostane inhibits the 3-beta-hydroxysteroid dehydrogenase enzyme system , thereby blocking aldosterone and cortisol production. The drug is currently marketed in Europe, and it is touted as the treatment-of-choice for pituitary-dependent HAC in dogs. Steroidogenesis inhibitors have been used primarily as pre-surgery treatments in people, rather than as long-term therapy, because the effects are eventually over-ridden by increasing ACTH. Trilostane was removed from the U.S. market in the 1990's. There are several reports of large clinical studies of the use of trilostane for treatment of dogs with HAC.7-9 . In the United States, most dogs with HAC are currently treated with mitotane, but when trilostane is re-introduced to the American market, should dogs be treated with this drug instead? The following points should be considered before a strong recommendation is made.
• Survival times with trilostane are no different from those with mitotane.10
• Sudden deaths, and deaths within a few days of starting therapy, have been reported with trilostane usage, while there are no reports of mitotane-induced death.11-13.
• Incidence of side effects with trilostane may be lower than with mitotane, but the side effects of mitotane are probably secondary to acute steroid withdrawal and are temporary.12
• Optimal dosing regimens for trilostane have not been established firmly, but twice-daily dosing is probably needed.8,14 Maintenance mitotane therapy usually requires twice or thrice-weekly dosing.
• Trilostane therapy is likely to more expensive than mitotane.
• Monitoring of trilostane therapy with the ACTH stimulation test may be difficult because ACTH can override the competitive inhibition of steroidogenesis.
• Adrenal tumors cannot be treated with trilostane, but they are often treated effectively with mitotane.15
• Because ACTH secretion continues in dogs with pituitary-dependent HAC, adrenal hyperplasia can progress despite trilostane therapy.
Trilostane may turn out to be the best treatment for pituitary-dependent HAC in the dog, but that has not been established by strong evidence. There are other steroidogenesis inhibitors available for use in the dog. Ketoconazole, for example, might be a better choice than trilostane because it inhibits multiple enzymes in the steroidogenesis pathway (see Figure 1) and also inhibits pituitary ACTH secretion. Like trilostane, ketoconazole is give daily or twice daily. Unlike trilostane, ketoconazole has not been studied extensively in dogs and is currently marketed in the United States.
REFERENCES
1. Van Liew CH, Greco DS. Salman MD. Comparison of results of adrenocorticotropic hormone stimulation and low-dose dexamethasone suppression tests with necropsy findings in dogs: 81 cases (1985-1998) J Am Vet Med Assoc 1997;211:322-5.
2.Kaplan AJ, Peterson ME, Kemppainen RJ. Effects of disease on the results of diagnostic tests for use in detecting hyperadrenocorticism in dogs.J Am Vet Med Assoc. 1995;207:445-51.
3. Benitah N, Feldman EC, Kass PH, Nelson RW. Evaluation of serum 17-hydroxyprogesterone concentration after administration of ACTH in dogs with hyperadrenocorticism.
J Am Vet Med Assoc. 2005;227:1095-101.
4. Ristic JM, Ramsey IK, Heath EM, Evans HJ, Herrtage ME. The use of 17-hydroxyprogesterone in the diagnosis of canine hyperadrenocorticism.
J Vet Intern Med. 2002;16:433-9.
5. Chapman PS, Mooney CT, Ede J, Evans H, O'Connor J, Pfeiffer DU, Neiger R. Evaluation of the basal and post-adrenocorticotrophic hormone serum concentrations of 17-hydroxyprogesterone for the diagnosis of hyperadrenocorticism in dogs.Vet Rec. 2003;153:771-5.
6. Behrend EN, Kemppainen RJ, Boozer AL, Whitley EM, Smith AN, Busch KA. Serum 17-alpha-hydroxyprogesterone and corticosterone concentrations in dogs with nonadrenal neoplasia and dogs with suspected hyperadrenocorticism.
J Am Vet Med Assoc. 2005;227:1762-7.
7. Braddock JA, Church DB, Robertson ID, Watson AD. Trilostane treatment in dogs with pituitary-dependent hyperadrenocorticism.
Aust Vet J. 2003;81:600-7.
8. Alenza DP, Arenas C, Lopez ML, Melian C. Long-term efficacy of trilostane administered twice daily in dogs with pituitary-dependent hyperadrenocorticism.
J Am Anim Hosp Assoc. 2006;42:269-76.
9. Behrend EN. Update on drugs used to treat endocrine diseases in small animals.
Vet Clin North Am Small Anim Pract. 2006;36:1087-105.
10. Barker EN, Campbell S, Tebb AJ, Neiger R, Herrtage ME, Reid SW, Ramsey IK. A comparison of the survival times of dogs treated with mitotane or trilostane for pituitary-dependent hyperadrenocorticism.J Vet Intern Med. 2005;19:810-5.
11. Chapman PS, Kelly DF, Archer J, Brockman DJ, Neiger R. Adrenal necrosis in a dog receiving trilostane for the treatment of hyperadrenocorticism.
J Small Anim Pract. 2004;45:307-10.
12. Kintzer PP, Peterson ME. Mitotane (o,p-DDD) treatment of 200 dogs with pituitary-dependent hyperadrenocorticism. J Vet Intern Med1995; 5:182-190.
13. Neiger R, Ramsey I, O'Connor J, et al. Trilostane treatment of 78 dogs with pituitary-dependent hyperadrenocorticism. Vet Rec 2002:799-804.
14. Bell R, Neiger R, McGrotty Y, Ramsey IK. Study of the effects of once daily doses of trilostane on cortisol concentrations and responsiveness to adrenocorticotrophic hormone in hyperadrenocorticoid dogs.Vet Rec. 2006;159:277-81.
15. Kintzer PP, Peterson ME. Mitotane treatment of 32 dogs with cortisol-secreting adrenocortical neoplasms. J Am Vet Med Assoc. 1994;205:54-61.