Delineating the cause of a bearded dragon's anorexia and weight loss
A contrast enema study helped clinicians detect an intestinal stricture in this exotic pet. See how this diagnostic step can help your reptile patients with similar signs.
Anorexia and constipation in reptiles can have multiple causes, including parasitism, ingestion of foreign objects, poor husbandry, neoplasia, and metabolic disorders.1,2 It is often difficult to determine the cause of these clinical signs in reptiles, and radiography can be a valuable diagnostic tool. The use of contrast radiography, usually an upper gastrointestinal (GI) barium contrast study, aids in the assessment of GI transit time and the detection of foreign bodies.3-5
In this article, we discuss the use of a different method of contrast radiography—a partial iohexol enema—to differentiate the large bowel from the small bowel and to diagnose an intestinal stricture in a bearded dragon.

PATIENT HISTORY
An adult intact male bearded dragon (Pogona vitticeps) weighing 419 g was presented to the Kansas State University Zoological Medicine Service for evaluation of a two-week history of anorexia and weight loss. Whole body radiography performed by the referring veterinarian had revealed a focal area of mixed opacity in the caudal coelomic cavity that seemed to be located in the intestinal tract.
An upper GI barium contrast study performed by the referring veterinarian had revealed contrast flowing normally through the stomach and small intestine but then pooling anterior to a heterogeneous mass in the distal GI tract. Small amounts of barium were able to bypass the mass and were excreted, although fecal output was markedly decreased.

VITAL STATS
INITIAL EVALUATION AND TREATMENT
On physical examination, the patient was emaciated but bright and alert; its body condition score was 2/5. Complete blood count and serum chemistry profile results showed mild anemia with a low hematocrit (14%; reference range = 19% to 40%) but were otherwise unremarkable.6,7
Because of the patient's poor body condition and anesthetic risk, conservative therapy was initiated to relieve the obstruction. Therapy consisted of warm-water enemas with sterile lubrication, oral liquids (water) delivered via an orogastric tube, fluids (45 ml/kg lactated Ringer's solution with 2.5% dextrose and 0.05 ml of a vitamin B complex administered subcutaneously), and warm-water soaks in a shallow bowl for 20 minutes three times daily so the patient could absorb water through the cloaca.
No clinical improvement was noted after 24 hours of medical therapy, and the mass was still palpable in the abdomen. Repeat survey radiographs confirmed the accumulation of heterogeneous material mixed with barium consistent with a GI impaction; it was unclear if the impaction involved the small or large intestine.
FLUOROSCOPY AND CONTRAST RADIOGRAPHY
A fluoroscopic evaluation was performed to determine the location of the mass. A pneumocolonogram obtained under fluoroscopy was attempted, using a size 8 red rubber catheter passed into the colon via the cloaca, but it was nondiagnostic.
Subsequently, a contrast enema using fluoroscopy was performed using 10 ml undiluted iohexol administered through the cloacal catheter. The contrast media passed through the descending colon into an irregularly narrowed area of the ascending colon.8 There was an obvious narrowing of the lumen of the small bowel aborad to the bolus of ingesta. No contrast material was able to pass through this area, which appeared to be at the ileocolonic junction (Figure 1). A stricture was suspected based on this radiographic study.

1. A contrast fluoroscopic image showing a stricture at the ileocolonic junction in a bearded dragon. Barium is pooling in the dilated ileum (I), iohexol is filling normal colon (C), and the stricture is visible in between. Note the narrowing at the colon (white arrowhead), the narrowing of the ileum (white box arrow), and the stricture (black arrows).
TREATMENT AND PATIENT OUTCOME
Because of the patient's poor quality of life and the stricture that did not resolve medically, an exploratory celiotomy was performed. The patient was premedicated with a combination of ketamine (10 mg/kg), medetomidine (0.1 mg/kg), and morphine (1.5 mg/kg) given intramuscularly and was then intubated with a 2-mm endotracheal tube and maintained on isoflurane with positive pressure ventilation.
During the surgery, thickening of the ileocolonic junction and a 3-mm band of white, fibrous tissue were noted; a large bolus of ingesta on the oral side of the fibrous band caused marked distention of the small intestines. The wall of the small intestine adjacent to the stricture was diffusely thin and friable, while the wall of the entire colon was thick and grossly appeared normal.
The vessels supplying the fibrous band and bolus were ligated using 5-0 polydioxanone (PDS) suture and bipolar cautery. The diseased tissue, about 9 cm long, was resected, and an anastamosis was performed using 5-0 PDS suture in a simple interrupted pattern.
Unfortunately, the anastamosis failed to pass a leak test, and the small intestine began to deteriorate around the sutures. The anastomosis was resected and another resection and anastamosis was performed while trying to minimize tissue handling. However, this site also leaked, and the small intestinal wall again began to deteriorate as before. Because of the grave prognosis, the patient was euthanized with 2 ml of pentobarbital injected into the ventral abdominal vein.
NECROPSY AND HISTOPATHOLOGIC EXAMINATION
Gross necropsy revealed no other lesions. The resected tissue was preserved in formalin and submitted for histopathologic evaluation.
The fibrous structure was determined to be a stricture at the ileocolonic junction, with extensive granulomatous inflammation in both the large and small intestines (Figure 2). Severe atrophy and ulceration of the small intestinal villi were noted, and the submucosa and tunica muscularis layers of the small intestine were expanded by sheets of foamy macrophages. No causal organisms were identified.
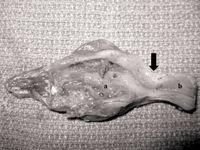
2. The resected lesion in a bearded dragon. Note that the dilated small intestine (a) and normal colon (b) are separated by a thick fibrous band of tissue at the ileocolonic junction (black arrow).
Possible causes of the stricture included a previous foreign body penetrating the wall of the intestine or an infectious cause of colitis that could not be identified with special stains. The thin and friable small intestine walls may have been attributable to the prolonged anorexia and anemia.
DISCUSSION
In this case, the partial (the colon was not completely distended) iohexol enema using fluoroscopy determined the location of the obstruction and identified it as a ileocolonic junction stricture. Contrast studies, usually upper GI studies using barium, have been used extensively to diagnose GI disease in reptiles.3,4 An iohexol enema was more advantageous in this patient than the upper GI study because the stricture completely occluded the lumen of the bowel, making it impossible to evaluate the colon using contrast medium delivered orally to the lesion. Although they were performed in conjunction with fluoroscopy in this case, plain radiographs can also be used for upper GI or enema contrast studies in reptiles.
There are several other instances in which an iohexol enema would be more advantageous than a barium upper GI study. For example, if a GI perforation was suspected, then barium would be contraindicated as it can cause life-threatening peritonitis.9 However, iohexol is water-soluble and much less irritating to the peritoneum, making it safer to use if a perforation is suspected.10 Also, if the case requires rapid diagnosis, a barium upper GI study would be inappropriate because the GI transit time of most reptiles is about 30 hours.3,11 A thorough barium contrast study that evaluates the entire GI tract takes several days of imaging, whereas a contrast enema, while only useful for the lower GI tract, can diagnose a lesion in just a few minutes.
Contrast radiographic procedures have frequently been replaced by endoscopy for the diagnosis of intestinal disease in domestic animals, but this technique is difficult in smaller reptile patients. Ultrasonography is often used to diagnose disease in the upper GI tract of mammals but less so for the colon because of acoustic shadowing of gas in the lumen.12
Determining the exact location of a GI lesion is of great importance when planning a surgery and in determining the prognosis. No difference in prognosis for large bowel surgery compared with small bowel surgery has been shown in dogs and cats. However, surgeries involving resection and anastamosis of the large bowel usually require a longer surgical time and longer hospitalization after surgery, which have been shown to correspond to increased morbidity.13 Thorough imaging is also important to help determine if there is more than one lesion that will require surgery, as animals undergoing more than one intestinal procedure have increased mortality.13
CONCLUSION
An iohexol enema is a valid and relatively simple procedure that is underused in diagnosing intestinal lesions in reptiles and is very useful when combined with an upper GI study. This imaging technique is easily applied in a private practice setting, where access to the more expensive advanced imaging techniques such as magnetic resonance imaging or computed tomography is limited. The use of contrast enemas should be encouraged when obtaining radiographs of reptiles with GI disease.
Acknowledgments
The authors thank Drs. Matt Sherwood and Marian Benitez (Department of Clinical Sciences, College of Veterinary Medicine, Kansas State University) for performing the surgery and Dr. Jamie Henningson (Department of Diagnostic Medicine & Pathobiology, College of Veterinary Medicine, Kansas State University) for providing the histopathology report.
Katie W. Delk, DVM, David S. Biller, DVM, DACVR, James W. Carpenter, DVM, DACZM, Department of Clinical Sciences, College of Veterinary Medicine, Kansas State University, Manhattan, KS 66506.
REFERENCES
1. Barten SL. Lizards. In: Mader DR, ed. Reptile medicine and surgery. 2nd ed. St. Louis, Mo: Saunders/Elsevier, 2006;683-695.
2. Büker M, Foldenauer U, Simova-Curd S, et al. Gastrointestinal obstruction caused by a radiolucent foreign body in a green iguana (Iguana iguana). Can Vet J 2010; 51(5):511-514.
3. Di Bello A, Valastro C, Staffieri F, et al. Contrast radiography of the gastrointestinal tract in sea turtles. Vet Radiol Ultrasound 2006;47:351-354.
4. Banzato T, Russo E, Finotti L, et al. Development of a technique for contrast radiographic examination of the gastrointestinal tract in ball pythons (Python regius). Am J Vet Res 2012;73(7):996-1001.
5. Smith D, Dobson H, Spence E. Gastrointestinal studies in the green iguana: technique and reference values. Vet Radiol Ultrasound 2001;42(6):515-520.
6. Gibbons P, Klaphake ME, Carpenter JW. Reptiles. In: Carpenter JW, ed. Exotic animal formulary. 4th ed. St. Louis, Mo: Elsevier, 2013;83-182.
7. Tamukai K, Takami Y, Akabane Y, et al. Plasma biochemical reference values in clinically healthy captive bearded dragons (Pogona vitticeps) and the effects of sex and season. Vet Clin Pathol 2011;40(3):368-373.
8. Banzato T, Selleri P, Veladiano IA, et al. Comparative evaluation of the cadaveric and computed tomographic features of the coelomic cavity in the green iguana (Iguana iguana), black and white tegu (Tupinambis merianae) and bearded dragon (Pogona vitticeps). Anat Histol Embryol 2013;24(6):453-460.
9. Karanikas ID, Kakoulidis DD, Gouvas ZT, et al. Barium peritonitis: a rare complication of upper gastrointestinal contrast investigation. Postgrad Med J 1997;73(859):297-298.
10. Rendano VT Jr. Radiology of the gastrointestinal tract of small animals. Can Vet J 1981;22(11):331-334.
11. Silverman S. Diagnostic imaging. In: Mader DR, ed. Reptile medicine and surgery. 2nd ed. St. Louis, Mo: Saunders/Elsevier, 2006;471-489.
12. Mai W. Imaging the stomach and intestine: pathologic aspect, in Proceedings. Atlantic Coast Vet Conf, 2008.
13. Wylie KB, Hosgood G. Mortality and morbidity of small and large intestinal surgery in dogs and cats: 74 cases (1980-1992). J Am Anim Hosp Assoc 1994;30:469-474.