Diagnosing and treating primary hypoparathyroidism in dogs and cats
In this article, we review the differential diagnoses in patients with hypocalcemia, the diagnostic testing to investigate clinical signs consistent with hypocalcemia, and the treatment of primary hypoparathyroidism.
In the preceding article, we discussed the pathophysiology of primary hypoparathyroidism and resulting hypocalcemia. The clinical signs of hypocalcemia are the same regardless of the underlying cause. In this article, we review the differential diagnoses in patients with hypocalcemia, the diagnostic testing to investigate clinical signs consistent with hypocalcemia, and the treatment of primary hypoparathyroidism.
DIFFERENTIAL DIAGNOSES
The various differential diagnoses in patients with hypocalcemia can be organized based on the degree of hypocalcemia and the prevalence of the underlying disease causing hypocalcemia. An important cause for a falsely low serum calcium concentration is laboratory error or improper sample anticoagulant (addition of EDTA, which chelates calcium).1
Common causes of low serum total calcium include hypoalbuminemia, renal failure, puerperal tetany, and acute pancreatitis.1,2 Less frequently, hypocalcemia occurs with ethylene glycol intoxication, administration of phosphate-containing enemas or intravenous sodium bicarbonate, soft tissue trauma, rhabdomyolysis, and primary hypoparathyroidism.1,2 Other causes of hypocalcemia include rapid intravenous administration of phosphates, dilution when calcium-free intravenous fluids are administered, intestinal malabsorption, starvation, citrated blood or plasma transfusions, hypovitaminosis D, thyroid tumors, cervical trauma or surgery, hypomagnesemia, aminoglycoside intoxication, and nutritional secondary hyperparathyroidism.1-3
Causes of hypocalcemia that have been identified in people but have not yet been clearly established in cats or dogs include pseudohypoparathyroidism (end-organ resistance to parathyroid hormone [PTH]), drug-induced hypoparathyroidism (most commonly from chemotherapeutic agents), sepsis, primary or metastatic bone tumors, hypercalcitoninism, and 131 I radiation damage.1,2,4
Primary hypoparathyroidism may be due to the absence or destruction of the parathyroid gland (as with surgical removal or immune-mediated disease, respectively). Transient hypoparathyroidism may occur with rapid correction of long-standing hypercalcemia (hypercalcemia causing temporary atrophy of the gland), as can occur with surgical removal or ablation of a parathyroid adenoma causing primary hyperparathyroidism.1,2 Thyroid surgery in both dogs and cats may result in removal or damage to the parathyroid glands. Thyroidectomy is the most common cause of hypoparathyroidism in cats.1,3 Parathyroid agenesis has been reported in a dog1 and was suspected in a cat.5
DIAGNOSIS
A serum chemistry profile may reveal results that are indicative of primary hypoparathyroidism. Primary hypoparathyroidism can be definitively diagnosed by measuring serum pth and ionized calcium concentrations.
Biochemical profile
In addition to ionized hypocalcemia, animals with hypoparathyroidism have relative or absolute hyperphosphatemia.1 In a case series of 37 dogs with hypoparathyroidism, the serum phosphorus concentrations were greater than the serum calcium concentrations in every case.1 Young animals normally have slightly higher phosphorus concentrations, and most laboratories include these values when establishing their reference ranges, resulting in higher reference ranges for phosphorus concentration.1,3 However, anorectic hypoparathyroid dogs may have relative phosphate depletion, which may or may not be reflected in the serum phosphorus concentration.1 The combination of hypocalcemia and hyperphosphatemia, with normal serum albumin, blood urea nitrogen, and creatinine concentrations, is indicative of primary hypoparathyroidism.1,2
Parathyroid hormone measurement
In the past 15 years, human PTH assays have been validated for use in dogs and cats.1,4,6-8 Before a validated PTH assay became available for use in animals, a histologic examination was used to confirm the diagnosis of primary hypoparathyroidism.6,9 With the advent of a reliable PTH assay, biopsy is no longer necessary to confirm hypoparathyroidism.
The definitive test for hypoparathyroidism is evaluating a serum PTH concentration and a concurrent ionized calcium concentration.1,4 With a normal functioning parathyroid gland, PTH should be increased in response to a low ionized calcium concentration.2 An animal with hypoparathyroidism will have an inappropriately low serum PTH concentration (undetectable to low-normal) with a low ionized calcium concentration.2,7 So it is essential to perform both tests on the same blood sample. Serum samples should not be kept at room temperature for longer than two hours before they are refrigerated or frozen.7,8 Contact the laboratory for guidelines on handling samples before collecting them because incorrectly handled samples may yield erroneously low results.8
TREATMENT
Table 1 provides an overview of the treatment recommendations for patients with primary hypoparathyroidism.

Table 1: Treatment of Primary Hypoparathyroidism
Initial treatment
Typically, affected animals are hospitalized for treatment because they require intensive care and frequent re-evaluation. Animals with tetany or seizure activity may respond to empirical administration of diazepam.1,3 With prolonged tetany, animals may become hyperthermic. As muscle relaxation occurs with treatment, a gradual normalization of body temperature occurs and additional cooling measures are typically not necessary.1-3 Serial monitoring of body temperature is recommended.3
When hypocalcemia has been established to be the cause of tetany, administer a slow intravenous bolus of a calcium salt.1,2 Ten percent calcium gluconate provides 9.3 mg/ml elemental calcium and is the preferred calcium salt because it is not irritating if accidentally injected perivascularly.1-3 The dosage of 10% calcium gluconate is 0.5 to 1.5 ml/kg (5 to 15 mg/kg) over 10 to 30 minutes, to effect.1-3,10 An alternative calcium salt is 10% calcium chloride, which contains 27.2 mg/ml elemental calcium.1,2 However, because of its higher calcium concentration, calcium chloride is more likely to cause severe tissue trauma and calcinosis cutis if injected perivascularly.1-3,11 The calcium salt must not be diluted in fluids that contain lactate, acetate, bicarbonate, or phosphates because calcium precipitation will occur, but 0.9% sodium chloride solution is appropriate.1-3
Monitoring an electrocardiogram (ECG) for potential cardiac arrhythmias is important during initial treatment for hypocalcemia because, when present, the arrhythmia may temporarily worsen. Various arrhythmias may occur with low ionized calcium concentrations, including bradycardia, tachycardia, and heart block.12 Because of the prolonged action potential in cardiac cells, the S-T and Q-T segments are often prolonged, with deep and wide T waves.1,3,12 Bradycardia, sudden elevation of the S-T segment, shortening of the Q-T interval, or ventricular premature complexes during administration all indicate cardiotoxicity from the calcium infusion,1-3 and the intravenous infusion should be temporarily stopped. When the ECG normalizes, reinstitute the infusion at a slower rate if further administration is needed to control tetany. The dosage provided above is a guideline only; the patient's response (resolution of neuromuscular signs) should guide individual dosing.1,3
Hypocalcemic animals may respond to administration of a calcium salt with only a slight increase in measurable calcium. Neuromuscular signs usually resolve when the total calcium concentration reaches 6 to 7 mg/dl or the ionized calcium concentration reaches 0.6 to 0.7 mmol/L. The immediate goal of treatment is to control the neurologic and neuromuscular signs, not to normalize the laboratory values.1,3 Neuromuscular signs may improve immediately, but complete resolution of nervousness and behavioral signs may take one to two hours while the extracellular calcium equilibrates with the cerebrospinal fluid.1,2,10,11 Attempting to normalize calcium concentrations too rapidly places the patient at risk for hypercalcemia, hyperphosphatemia, and subsequent soft tissue mineralization because of deposition of calcium phosphate complexes (particularly in the skin), and renal parenchymal mineralization or calculus formation.1,3,13 The mass law effect, however, acts to lower the serum phosphorus concentration to compensate as the serum calcium concentration rises, so mineralization is rarely a clinical concern.1
Transitional treatment
At a minimum, monitor the serum calcium concentration daily during treatment.2,3 The duration of daily evaluation depends on the long-term therapy selected and status of the animal. The short-term goal is to maintain a total serum calcium concentration between 8 to 9 mg/dl until long-term oral therapy can begin.1,3 The initial dose of calcium salt administered to control clinical signs can last anywhere from one to 12 hours.1,2,4 At this stage, animals with hypoparathyroidism generally require additional parenteral calcium salt administration. Administration of a vitamin D analogue and oral calcium supplementation should also be initiated.
Oral therapy requires a minimum of one to three days of therapy before becoming effective. During this transitional period, parenteral calcium salts are given as either repeated intravenous boluses, subcutaneously (calcium gluconate only), or as a constant-rate infusion (CRI).1-3 When an animal requires additional intravenous fluid therapy support, avoid alkalinizing fluids since they decrease the serum ionized calcium concentration, exacerbating hypocalcemia.1,2 The length of therapy required is case-dependent. Hypoparathyroidism secondary to unilateral thyroidectomy may not require lifelong therapy. Bilateral parathyroidectomy resulting from thyroidectomy requires lifelong treatment as described in the section on long-term management.
Repeated intravenous boluses of calcium salts are the least optimal method of hypocalcemic treatment because the boluses cause peak and trough levels of extracellular calcium.1-3 Subcutaneous calcium salt administration is easy, inexpensive, and generally effective.1,3 Dosing guidelines vary, but the same dose of 10% calcium gluconate solution that was used initially to control tetany can be diluted 1:1 to 1:5 with sterile 0.9% sodium chloride solution and administered subcutaneously every six to eight hours.1-3,10,13 Alternatively, diluted calcium gluconate can be administered subcutaneously at 60 to 90 mg/kg/day, divided into three or four doses.1,2 However, 10% calcium chloride should not be administered subcutaneously because of tissue irritation and saponification of fat.1,2,10 Potential side effects, such as irritation and calcinosis cutis, do exist even with the use of diluted 10% calcium gluconate.13,14 If the calcium concentration remains stable after 48 hours of subcutaneous therapy, the frequency of parenteral calcium salt administration can be reduced to every 12 hours and then slowly withdrawn as the oral treatments begin to take effect.1-3
Another option to maintain steady extracellular calcium concentration is a CRI of 10% calcium gluconate at 2.5 to 10 mg/kg/hr (or 60 to 90 mg/kg/day).1-3,10 Some authors recommend administering intravenous fluids at a maintenance rate (60 ml/kg/day) with 10, 20, or 30 ml of 10% calcium gluconate added to a 250-ml fluid bag to create a 1-, 2-, or 3-mg/kg/hr elemental calcium infusion.1,2 The higher rate of infusion is used for more severely hypocalcemic patients.2 In patients receiving calcium as a CRI, measure the serum calcium concentration (either total or ionized) every eight to 12 hours to assess efficacy and avoid hypercalcemia.10 As the oral treatments begin to take effect, gradually withdraw CRI therapy similar to subcutaneous therapy.2 At this point, administering an oral vitamin D analogue and oral calcium salts should maintain serum calcium concentration.
Long-term management
Lifelong administration of a vitamin D analogue is the mainstay of treatment for primary hypoparathyroidism.1-4,9 In patients with primary hypoparathyroidism, the vitamin D concentration is also decreased. For the intestines to have normal absorption of dietary and supplemental oral calcium, vitamin D must be present in sufficient quantity. The goal of oral supplementation is to maintain the total serum calcium concentration between 8 and 9.5 mg/dl, which is just below the normal range, to avoid signs of hypocalcemia yet minimize the risk of developing hypercalcemia.1-3,10 Initial supplementation with oral calcium salts is often required for the first few months.
Several vitamin D analogues are available (Table 2). The most commonly prescribed are ergocalciferol (vitamin D2), dihydrotachysterol, and calcitriol (1,25-dihydroxycholecalciferol).1,2 The dosages of these analogues vary widely, and the onset of effect to increase serum calcium concentration varies by preparation. A minimum of 24 to 96 hours of therapy is needed before there is an effect, and hypercalcemia commonly develops.1,2 Hypercalcemia causes serious and even fatal complications that include acute or chronic renal failure. Counsel owners regarding the signs of hypercalcemia (polydipsia, anorexia, vomiting, and depression), and instruct owners to discontinue all supplementation and seek veterinary care if these signs are noted.1,2,9,10 Furthermore, regular monitoring of the total serum calcium concentration is crucial for the management of hypoparathyroidism.
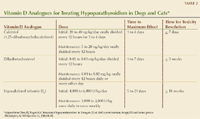
Table 2: Vitamin D Analogues for Treating Hypoparathyroidism in Dogs and Cats*
When the patient is stabilized, discharged from the hospital, and receiving oral medications, measure the total serum calcium concentration at least weekly.2 When calcium concentrations remain consistently within the desired range, these re-evaluations may occur every three to four months, though more frequent re-evaluation allows better control of the calcium concentration and minimizes the risk of complications.1-3
Vitamin D dose increases of 10% to 20% are indicated when the total serum calcium concentration is below the ideal value.2 When the calcium concentration multiplied by the phosphorus concentration is > 60 to 70, there is an increased risk for soft tissue mineralization. In growing animals, it is also prudent to monitor the serum phosphorus concentration to identify and address a calcium-phosphorus product > 60 to 70.5 Treatment would involve medications to lower either the calcium concentration or phosphorus concentration or both. Additionally, if the patient is an intact female, ovariohysterectomy is indicated because in people, estrogen, pregnancy, and lactation complicate regulation of serum calcium concentration.9
Vitamin D analogues
Ergocalciferol. The benefits of ergocalciferol (vitamin D2) include wide availability and low cost, but its unpredictability in an individual patient and long half-life make it less than ideal.1-3,9 Initially, large doses (4,000 to 6,000 U/kg orally every 24 hours) are administered.1-3,10 Large doses may be required because of the lack of PTH effect, which normally aids in the conversion of ergocalciferol to a more active analogue.1-3,9 Ergocalciferol is fat-soluble and distributes slowly throughout the body fat stores.1-3 An increase in serum calcium concentration may become apparent five to 21 days after initiation of therapy.1-3,10 Parenteral calcium supplementation can usually be discontinued one to five days after the start of ergocalciferol treatment.1,3 Once the serum calcium concentration is stable, the dose may be gradually decreased to between 1,000 and 2,000 U/kg once daily to once weekly.1-3,10
Because the dose of ergocalciferol required by individual patients varies markedly, there is a risk for the development of severe hypercalcemia.1,2 If a patient becomes hypercalcemic, the condition may persist for one to 18 weeks because of ergocalciferol's long half-life and extensive fat distribution in the body.1,2 If hypercalcemia develops, treat it immediately by discontinuing ergocalciferol and calcium supplementation. If hypercalcemia is refractory, consider administering intravenous fluid therapy with 0.9% sodium chloride solution, a noncalcium-retaining diuretic such as furosemide, and corticosteroids.1 Corticosteroids increase calciuresis, reduce intestinal absorption of calcium, and inhibit calcium resorption.
Dihydrotachysterol. Dihydrotachysterol is a readily available synthetic vitamin D analogue that is superior to ergocalciferol because of its potency, rapid onset of action, and half-life.1-3,10 For comparison, 1 mg of dihydrotachysterol is equivalent to 120,000 U of ergocalciferol.1,3 Also because of its molecular structure, dihydrotachysterol is not stored in fat to the same degree that ergocalciferol is stored.1,2 In the liver, dihydrotachysterol undergoes 25-hydroxylation, forming a molecule similar to calcitriol; however, it is less effective because it lacks a 3-beta-hydroxyl group.2,15 The onset of action of dihydrotachysterol is one to seven days, and it is eliminated from the system more rapidly after discontinuation, usually in four to 14 days (although it may last 30 days),1-3,10 making it safer and easier to adjust the dose than ergocalciferol.3,10 However, dihydrotachysterol costs more than ergocalciferol. Some patients do not respond to the tablet or capsule formulations of dihydrotachysterol but respond to a liquid formulation.1,3 Other animals are completely unresponsive to dihydrotachysterol and require therapy with calcitriol.1
The initial dosage of dihydrotachysterol is 0.03 mg/kg/day orally divided every 12 hours until an effect is demonstrated (usually two to three days), then 0.02 mg/kg/day orally divided every 12 hours for two to three days, followed by a maintenance dosage of 0.01 to 0.02 mg/kg/day orally divided every 12 hours and given every one to two days (one half is administered in the morning, the other half in the evening; some patients require this every day, while others require it every other day).2,3 Although superior to ergocalciferol, there remains a substantial dose variation in the amount of dihydrotachysterol required for individual patients.2
Calcitriol. Calcitriol (vitamin D3) is the ideal vitamin D analogue because of its potency and the precise control of serum calcium it allows.1,2,9,10 Calcitriol does not require activation and is not stored within the body.3,10 It has a rapid onset of action (one to four days) and a short half-life (less than a day), allowing for frequent dose adjustments.1-3,10 Because of this, there is minimal concern regarding induction of hypercalcemia if an animal is monitored as recommended. If hypercalcemia develops, the calcitriol dose can be reduced or discontinued until the hypercalcemia resolves.1-3,10
Expense is the major disadvantage of this drug.11 Additionally, calcitriol is available in only two tablet sizes (0.25 and 0.5 μg).2 To make small dosage adjustments, a liquid formulation is helpful.1,2 The loading dosage of calcitriol is 20 to 40 ng/kg/day orally divided for two to four days, followed by a maintenance dosage of 5 to 20 ng/kg/day divided every 12 hours.1,2 Some authors recommend up to 30 to 60 ng/kg/day.10 The dose may be divided to maximize its priming effects on the intestinal epithelial cells to transport calcium.2 Parenteral formulations of calcitriol are available when the oral formulations are ineffective or not well-tolerated.1
Oral calcium salt supplementation
The final component of long-term treatment of primary hypoparathyroidism at home is the administration of an oral calcium salt supplement (carbonate, lactate, gluconate, and chloride), given concurrently with the vitamin D analogue.1,3,10 In the immediate post-tetany period, high quantities of calcium in the gut increase calcium absorption independent of the presence of a vitamin D analogue.2,3 Later oral calcium supplementation also provides the necessary calcium on which vitamin D analogues function.1-3 The dosage for oral elemental calcium in dogs is 1 to 4 g/day (in cats 0.5 to 1 g/day),1,3 or more specifically 25 to 50 mg/kg or 100 to 500 mg/kg,2,10 divided three to four times daily. Commercially made pet foods contain sufficient calcium for animals with normal calcitriol concentrations.1-3 Therefore, oral calcium supplementation can be slowly reduced over two to four months once the vitamin D analogue administered has stabilized the serum calcium concentration.1-3 If hyperphosphatemia persists, oral calcium supplementation may be continued as a phosphate binder.2
Several calcium supplements are available that can be used during long-term management of primary hypoparathyroidism. In people, calcium carbonate is the treatment of choice because it has a relatively high calcium concentration (40%), which requires administration of fewer pills; it is widely available as over-the-counter antacids; it is inexpensive; and it does not cause gastric irritation.1-3 One disadvantage of calcium carbonate is that it may cause an alkalemia, which may aggravate hypocalcemia.1,3 Despite this, calcium carbonate is used frequently in veterinary medicine. Calcium gluconate (10% available calcium) and lactate (13%) contain less elemental calcium than calcium carbonate, so more tablets must be administered to achieve the same effect.1-3 Calcium chloride tablets contain more elemental calcium (27%) but are irritating to the gastric mucosa and should be given with food.1-3
Other therapeutic options used in human medicine
Oral vitamin D and calcium supplementation does not completely address the lack of PTH because hypercalciuria will persist.2,16 People with long-term management of hypoparathyroidism may develop nephrocalcinosis, urolithiasis, and decreased renal function.2 It has not been established that these complications occur in animals, but this appears possible.2 In people with hypoparathyroidism, thiazides are administered to reduce hypercalciuria and may decrease the necessary dose of vitamin D analogue through increased renal retention of calcium.2,16 It is not known if thiazide diuretics would have a similar effect in dogs or cats.2
In people, daily subcutaneous administration of PTH can be effective in maintaining serum calcium concentrations.2 Canine and human PTH are fairly homologous, and an immune response may not be mounted by dogs receiving human PTH.2 However, human PTH is expensive and not readily available, and eventual formation of anti-PTH antibodies is a foreseeable complication.1,9,14
PROGNOSIS
The prognosis for naturally occurring primary hypoparathyroidism depends on the dedication of the owner to manage the patient and the vigilance of the veterinarian to monitor the patient's status.1,3 In uncomplicated cases with appropriate care, the prognosis can be excellent.1,3 Normal life expectancy is reported by some authors.1,3 Others state that quality and length of life have yet to be determined.2
Conclusion
Owner awareness is essential for early detection of the clinical signs of both hypocalcemia and hypercalcemia. Patients with primary hypoparathyroidism present with clinical signs consistent with hypocalcemia that are indistinguishable from other causes of hypocalcemia. The most common presenting complaints are tetany, seizures, and behavioral changes. Evaluation of a complete biochemical profile and serum pth concentration in combination with a serum ionized calcium concentration is essential to confirm the diagnosis of primary hypoparathyroidism.
Initial treatment consists of intravenous calcium salts. After initial stabilization, the patient may be transitioned to an oral vitamin D analogue, which will need to be administered for the rest of its life, and oral calcium supplementation, at least temporarily. Regularly measuring total serum calcium concentration and monitoring the patient for clinical signs of hypocalcemia or hypercalcemia are paramount. The frequency of these evaluations will decrease from weekly to monthly, or less, as the patient's condition stabilizes. With the proper care, primary hypoparathyroidism has a good to excellent prognosis.
Beth L. McElravy, DVM
Jill D. Brunker, DVM, DACVIM
Department of Small Animal Internal Medicine
College of Veterinary Medicine
Oklahoma State University
Stillwater, OK 74078
REFERENCES
1. Feldman EC, Nelson RW. Hypocalcemia and primary hypoparathyroidism. In: Canine and feline endocrinology and reproduction. 3rd ed. Philadelphia, Pa: Elsevier Science, 2004;716-742.
2. Chew DJ, Nagode LA. Treatment of hypoparathyroidism. In: Bonagura JD, ed. Kirk's current veterinary therapy XIII small animal practice. Philadelphia, Pa: WB Saunders Co, 2000;340-345.
3. Feldman EC. Disorders of the parathyroid glands. In: Ettinger SJ, Feldman EC, eds. Textbook of veterinary internal medicine: diseases of the dog and cat. 6th ed. St. Louis, Mo: Elsevier Saunders, 2005;1508-1535.
4. Bruyette DS, Feldman EC. Primary hypoparathyroidism in the dog. J Vet Intern Med 1988;2:7-14.
5. Bassett JR. Hypocalcemia and hyperphosphatemia due to primary hypoparathyroidism in a six-month-old kitten. J Am Anim Hosp Assoc 1998;34:503-507.
6. Waters CB, Scott-Moncrieff JCR. Hypocalcemia in cats. Compend Cont Educ Vet Pract 1992;14:497-507.
7. Torrance AG, Nachreiner R. Intact parathyroid hormone assay and total calcium concentration in the diagnosis of disorders of calcium metabolism in dogs. J Vet Intern Med 1989;3:86-89.
8. Barber PJ. Disorders of the parathyroid glands. J Feline Med Surg 2004;6:259-269.
9. Sherding RG, Meuten DJ, Chew DJ, et al. Primary hypoparathyroidism in the dog. J Am Vet Med Assoc 1980;176:439-444.
10. Dhupa N, Proulx J. Hypocalcemia and hypomagnesemia. Vet Clin North Am Small Anim Pract 1998;28:587-608.
11. Greco DS. Endocrine emergencies. Part II. Adrenal, thyroid, and parathyroid disorders. Compend Cont Educ Vet Pract 1997;19:27-39.
12. Church D. Electrolyte disorders. In: Ettinger SJ, Feldman EC, eds. Textbook of veterinary internal medicine: diseases of the dog and cat. 6th ed. St. Louis, Mo: Elsevier Saunders, 2005;236-238.
13. Schaer M, Ginn PE, Fox LE, et al. Severe calcinosis cutis associated with treatment of hypoparathyroidism in a dog. J Am Anim Hosp Assoc 2001;37:364-369.
14. Ruopp JL. Primary hypoparathyroidism in a cat complicated by suspect iatrogenic calcinosis cutis. J Am Anim Hosp Assoc 2001;37:370-373.
15. Allen TA, Weingand K. The vitamin D (calciferol) endocrine system. Compend Cont Educ Vet Pract 1985;7:482-488.
16. Potts, JT. Diseases of the parathyroid gland and other hyper- and hypocalcemic disorders. In: Braunwald E, Fauci AS, Kasper DL, et al., eds. Harrison's principles of internal medicine. 15th ed. New York, NY: McGraw-Hill, 2004;2205-2226.







