Diagnostic testing offers early detection of renal disease
Dr. Greg Grauer identifies several options practitioners can use to detect the early stages of renal disease through routine diagnostic testing.
Improving renal function in dogs and cats with chronic renal failure (CRF) is often not possible inasmuch as the renal lesions and associated dysfunction are usually irreversible and progressive.

Diagnostic testing offers new options for detecting patients in the early stages of renal disease. An annual examination, that includes a complete blood count serum biochemistry and urinalysis, is a good method for detecting renal function.
Unfortunately, due to the large functional renal reserve and the compensatory hypertrophy of remaining nephrons, clinical signs and laboratory data compatible with renal failure are not present until greater than 75-85 percent of all nephrons are nonfunctional. Once CRF is established, management options are limited and usually directed at reducing clinical signs and attempting to slow disease progression.
Early detection of renal disease, prior to the onset of failure, should improve our ability to alter the course of the disease.
Clinical signs
Most CRF, unless the underlying lesions are familial, occurs in middle- to older-aged dogs and cats. An annual health examination, that includes a complete blood count, serum biochemistry profile, and urinalysis, is one of the best ways to detect declining renal function (Table 1). Special attention should be paid to decreases in appetite, body weight, packed cell volume, and urine specific gravity.

Table 1: Historical, clinicopathologic findings that may be associated with early renal disease in dogs and cats
Conversely, increases in serum urea nitrogen, creatinine, and phosphorus, or urinary excretion of protein may signal the onset of renal disease. Plotting the inverse of the serum creatinine concentration versus time can demonstrate a decrease in renal excretory function (Table 2,). Dogs and cats may also become more susceptible to bacterial urinary tract infections as their ability to concentrate urine decreases and the antibacterial properties of their urine decrease. If any of the above parameters suggest the possibility of renal disease, an ultrasound examination should be used to evaluate kidney tissue architecture. Pyelectasia (dilatation of the renal pelvis compatible with pyelonephritis) (Photo 1), renoliths (Photo 2), and renal cortical fibrosis can be demonstrated by ultrasound. Percutaneous or ultrasound-guided renal biopsy can be used to confirm or further define renal cortical disease.

Table 2: A hypothetical 1/serum creatinine concentration vs. time curve demonstrating declining renal function.
Azotemia and glomerular filtration rate
Azotemia is defined as increased concentrations of urea and creatinine in the blood. The interpretation of serum urea nitrogen and creatinine concentrations as a measure of renal function requires a knowledge of the production and excretion of these substances. Urea is synthesized in the liver from ammonia, which is in turn generated from the catabolism of ingested and endogenous proteins. Urea production is increased in the settings of high dietary protein intake and upper gastrointestinal tract hemorrhage. Conversely, urea production is decreased in the settings of a low dietary protein intake, decreased hepatic function, or decreased delivery of ammonia to the liver (e.g., portosystemic shunt). Urea nitrogen is excreted by glomerular filtration. Subsequent tubular reabsorption of urea nitrogen decreases the amount excreted by returning the waste product to the blood stream.
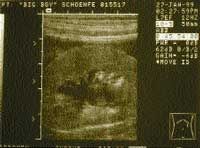
Photo 1: Ultrasound image from a canine kidney demonstrating dilatation of the renal pelvis (pyelectasia) compatible with pyelonephritis. (Courtesy of Dr. David Biller, Kansas State University).
Tubular reabsorption of urea is increased when tubular flow rates and volumes are decreased. Conversely, the tubular resorption of urea is decreased and excretion increased in the presence of diuresis.
Creatinine is formed by the nonenzymatic metabolism of creatine and phosphocreatine in muscle. Creatinine production is relatively constant and proportional to muscle mass; animals with a large muscle mass produce more creatinine each day than do animals with a small muscle mass. In comparison with the urea nitrogen, the creatinine concentration is relatively unaffected by the dietary protein level; however, serum creatinine concentrations can increase after the ingestion of meat and subsequent increased absorption of creatinine from the gastrointestinal tract. Creatinine is excreted via glomerular filtration.
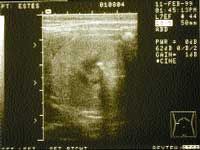
Photo 2: Ultrasound image from a canine kidney demonstrating a renouroliths. (Courtesy of Dr. David Biller, Kansas State University).
Serum urea nitrogen and creatinine concentrations provide a crude index of the glomerular filtration rate (GFR). Inasmuch as the creatinine concentration is influenced by fewer extrarenal variables and creatinine is not resorbed by the renal tubules, the serum creatinine concentration is a better index of GFR than the serum urea nitrogen. Nevertheless, azotemia resulting from impaired renal function is not detectable until relatively late in the disease process. Therefore measurement of GFR can provide more accurate information about renal excretory function than the serum creatinine and urea nitrogen concentrations, especially early in renal disease before 75-85 percent of the nephrons are nonfunctional.
Plasma clearance of iohexol, an iodinated radiographic contrast agent, can provide a reliable estimate of GFR in dogs and cats. After a single, intravenous injection of iohexol, plasma clearance of the substance is calculated as the quotient of the administered dose divided by the area under the plasma concentration versus the time curve. The area under the plasma concentration versus the time curve is determined from several plasma iohexol concentrations obtained after the initial injection. The plasma clearance of iohexol is then correlated with exogenous creatinine clearance by linear regression analysis.
Calculation of iohexol clearance does not require urine collection so the procedure is less labor intensive and invasive compared with creatinine clearance techniques. Plasma concentrations of iohexol can be measured and GFR calculated at specialized reference laboratories.
Renal scintigraphy
Renal scintigraphy using technetium 99m–labeled compounds also allows the GFR to be evaluated and is available at many university teaching hospitals and major referral centers. This is a quick noninvasive method that does not require urinary catheterization and has the advantage of being able to quantitatively evaluate individual kidney function. Disadvantages of this procedure include its limited availability, exposure of the animal to radioisotopes, and the need for radioisotope disposal.
Proteinuria
Proteinuria is routinely detected by semi-quantitative methods, including the dipstick colorimetric test and the sulfosalicylic turbidimetric test. Proteinuria detected by these semi-quantitative methods should always be interpreted in light of the urine specific gravity. For example, a 2+ proteinuria with a 1.010 urine specific gravity is suggestive of a much greater urine protein loss on a 24-hour basis than is the same 2+ proteinuria with a 1.040 urine specific gravity. Because the urine protein concentration is frequently increased in animals with lower urinary tract inflammation or hemorrhage, proteinuria should also be assessed in the context of urine sediment changes indicative of inflammation or hemorrhage (e.g., bacteria and increased numbers of white and red blood cells and epithelial cells in the urine sediment). The occurrence of persistent proteinuria with normal urine sediment analyses (an exception may be the presence of hyaline casts) is suggestive of glomerular disease.
When glomerular proteinuria is suspected, urine protein excretion should be quantified. This helps evaluate the severity of renal lesions as well as assess the response to treatment or the progression of disease. The urine protein/creatinine ratio (UP/C) in canine and feline urine samples has been shown to accurately reflect the quantity of protein excreted in the urine over a 24-hour period and has greatly facilitated the diagnosis of glomerular disease in small animals. A UP/C of less than 1 is considered normal in dogs and cats, although in several studies normal animals have UP/Cs less than 0.2-0.5. A complete urinalysis should always be performed before or along with determination of the UP/C, because hematuria or pyuria may indicate the presence of significant nonglomerular proteinuria. If there is evidence of inflammation (e.g., pyuria, bacteriuria or hematuria), the protein concentration should be measured again after successful treatment of the inflammatory disorder. The UP/C cannot be used to differentiate glomerular proteinuria from proteinuria associated with lower urinary tract inflammation or hemorrhage.
Recently an antigen capture ELISA test for the detection of low levels of albumin in canine urine (microalbuminuria) has become commercially available (E.R.D.-Screen, Heska Corporation).
Microalbuminuria is defined as a urine albumin concentration between 1.0 and 30 mg/dl. These are concentrations too small to be routinely detected by standard dipstick screening tests. It is interesting to note that the presence of microalbuminuria has been shown to be an accurate predictor of subsequent renal disease in human beings with both systemic hypertension and diabetes mellitus and it has also been observed in human beings with systemic diseases that are associated with glomerulopathy. Studies in dogs have shown the prevalence of microalbuminuria in apparently healthy dogs and those seeking veterinary care to be 19 percent and 36 percent, respectively. In soft-coated wheaten terriers genetically predisposed to developing glomerular disease, the prevalence of microalbuminuria was 76 percent. In another study, microalbuminuria developed in 100 percent of dogs with experimentally induced heartworm disease.
Further study is necessary to determine if microalbuminuria is an accurate predictor of overt proteinuria and renal disease in dogs and cats. If microalbuminuria does predict overt proteinuria and/or renal disease, this early detection tool should significantly increase our ability to alter renal disease progression.
Urine concentrating ability
The kidneys maintain body fluid composition and volume by resorbing water and solutes from the glomerular filtrate. The resorption of solute in excess of water results in the formation of dilute urine.
Conversely, the resorption of water in excess of solute results in the formation of concentrated urine. For concentrated urine to form, antidiuretic hormone (ADH) must be produced and released, and the renal tubules must be responsive to the ADH. For the latter to occur, renal medullary hypertonicity must be present and at least one third of the total nephron population must be functional. The animal's hydration status, serum urea nitrogen and creatinine concentrations, and current medications must be known in order to correctly interpret random urine specific gravity values. Normal dogs and cats should produce hypersthenuric urine (> 1.030-1.035) in response to detectable dehydration. Multiple urine specific gravities that are consistently isosthenuric (1.008 - 1.012) or minimally concentrated (> 1.012 but <1.030-1.035) can be associated with decreased renal function. Water deprivation testing should not be performed in patients with suspected renal dysfunction, as dehydration may exacerbate existing renal lesions.
Ultrasonography
Ultrasonography is used to evaluate renal tissue architecture if kidney abnormalities have been revealed by physical examination (e.g., abnormal kidney size or shape), clinicopathologic findings (e.g., azotemia or proteinuria), or survey radiographs (e.g., abnormal kidney size, shape, or opacity or nonvisualization of a kidney). Normally the renal cortex is hypoechoic compared with the spleen, liver, and the renal medulla. In comparison, the renal pelvis and diverticula are relatively hyperechoic. Decreased echogenicity of the renal cortices can be observed in patients with acute tubular necrosis, polycystic kidney disease, abscesses, and the renal edema associated with acute renal failure. Conversely, relatively hyperechoic renal cortices are associated with end-stage renal failure, nephrocalcinosis, amyloidosis, feline infectious peritonitis, and calcium oxalate nephrosis secondary to ethylene glycol ingestion. Glomerular and tubulointerstial disease can show a normal or hyperechoic echotexture depending on chronicity.

Suggested Readings
Renal lymphoma can make the renal cortices appear hypoechoic or hyperechoic. Hydronephrosis and hydroureter are easily diagnosed by ultrasonography and in cases of pyelectasia; fluid for culture and cytologic analysis can be aspirated by ultrasound guidance. Resistance to renal blood flow (resistive index) can be calculated with the use of color flow doppler, and is increased in several renal diseases.