How to perform and interpret dermatophyte cultures
Use this guide to maximize your success with this indispensable in-house test.
Dermatophyte cultures can be challenging to perform and interpret correctly. However, knowing how to best collect samples for culture, select and incubate culture media, and identify media culture changes and fungal colony morphology will help you avoid a misdiagnosis.
COLLECTING SAMPLES FOR CULTURE
Hair pluck
To obtain samples for dermatophyte culture, use a sterile hemostat to pluck hairs from around the periphery of a newly formed or expanding skin lesion, avoiding areas that may have been recently medicated. Ideal hairs to select are those in areas of active crusting and hairs that appear damaged or misshapen.1
Toothbrush technique
Hair plucks can potentially miss infected hairs and may not sample infected epithelium adequately, so it is ideal to also obtain samples using the Mackenzie brush technique. With this technique, a new toothbrush is removed from its packaging and is rubbed gently over the suspect area, including the skin and haired margins of alopecic or scaly lesions (Figure 1).1 Brush the unaffected area first, and then brush the lesions to avoid spreading spores to unaffected areas and to avoid losing spores from affected areas. Then gently embed the toothbrush bristles into the fungal culture media (Figure 2), taking care not to embed the bristles too deeply, which risks displacing the culture media when the bristles are removed. Use a sterile hemostat to remove hair and debris caught among the bristles, and place the material on the culture medium surface.

Figure 1. The Mackenzie brush technique is used to collect samples for dermatophyte culture.
The Mackenzie brush technique is helpful to screen for asymptomatic carriers and to obtain samples from animals undergoing antifungal treatment in which skin lesions have clinically resolved. In these cases, stroke the toothbrush over the entire body, concentrating especially on areas with prior lesions and, in cats, on the face, ears, and paws. It is recommended to brush for one minute or to brush the length of the animal 10 times.2 In animals undergoing antifungal therapy, repeat cultures every two or three weeks, and continue treatment until two negative culture results are obtained.2

Figure 2. The toothbrush bristles have been gently pressed onto the fungal culture media.
In cases of suspected onychomycosis, the toothbrush can be used on the affected claw fold. Additionally, samples of claw fold fur can be obtained with a sterile hemostat, and the proximal affected nail can be sampled by using a scalpel blade to shave off small pieces of keratin. (Precleaning the nail with alcohol is recommend to help reduce accumulated saprophytic or environmental fungal organisms.) If an avulsed claw is considered for fungal culture, discard the distal part of the nail, and obtain samples by scraping the proximal concave surface of the claw.1
Toothbrushes can be obtained inexpensively in bulk from dollar stores or from online distributors. They can be used once and discarded or gas sterilized for repeated use.
SELECTING AND INCUBATING CULTURE MEDIA
Dermatophyte test medium contains Sabouraud's dextrose agar with cycloheximide, gentamicin, and chlortetracycline as antifungal and antibacterial agents to retard the growth of contaminant organisms. The pH indicator phenol red is also added.
Dermatophytes preferentially metabolize protein in the culture medium, releasing alkaline metabolites that turn the yellow fungal culture medium to red at the same time the dermatophyte colony appears. Most other fungi initially use carbohydrates and produce acidic metabolites; these saprophytic fungi can eventually consume protein and cause media color change, but it occurs several days after fungal growth appears.1,3 Daily observation and logging of fungal growth correlated with media color change is, thus, important in correctly interpreting dermatophyte test medium culture results.
Culture plates are recommended over vials, as the vial openings are usually too narrow to pass toothbrush heads for inoculation or to easily sample fungal colonies for microscopic analysis.4 To facilitate fungal sporulation and identification, it may be helpful to use a dermatophyte test medium plate that has a separate area of plain Sabouraud's agar or rapid sporulation medium, which does not contain inhibiting agents. For example, the Dermatoplate-Duo (Vetlab Supply) culture plate has dermatophyte test medium on one side and enhanced sporulation agar on the other side.
According to recommendations from a fungal culture media manufacturer, culture media should be stored at 36 to 77 F (2 to 25 C) and protected from light before inoculation.5 The plates should be warmed to room temperature (77 to 86 [25 to 30 C]) before inoculation. Before and during the inoculation procedure, the plates should be handled in a manner that minimizes exposure of the media to the environment. Do not use expired plates or plates that exhibit drying, cracking, discoloration, microbial contamination, or other signs of deterioration. Excessive condensation may appear in plates that have been damaged by exposure to temperature extremes.5
Fungal cultures should be incubated at room temperature (77 to 86 F [25 to 30 C]) with 30% humidity.1,5 If room temperature is not maintained, use an incubator, or send the samples to a reference laboratory for culture.4
Most organisms will appear within seven to 10 days; however, plates should be kept for 21 days, especially when no growth is seen initially or when the sample has been obtained from a pet receiving antifungal therapy. According to a fungal culture media manufacturer, dermatophyte culture plates may be incubated in full light, although some authors recommend incubation in the dark to avoid UV light-induced inhibition of fungal growth.1,5 In dry climates, it is suggested that plates be placed in plastic bags or containers to prevent dehydration of the media, which can inhibit organism growth.5 After 48 to 72 hours, begin examining the plates daily for characteristic media color changes and fungal growth.
IDENTIFYING DERMATOPHYTES
Understanding macroscopic fungal colony morphology is an important first step in determining whether a dermatophyte is present. Microsporum and Trichophyton species—the most common dermatophytes in dogs and cats—are white, light yellow, tan, or buff-colored cottony-to-powdery-appearing colonies (Figures 3 & 4). Dermatophyte colonies are never black, green, or gray.
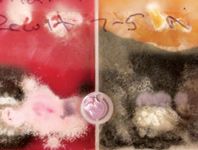
Figure 3. The dermatophyte culture plate exhibits Microsporum canis growth (the white-to-pale-yellow fungal colonies at the top of the culture plate) that is at risk of being overgrown by the gray saprophytic fungal colonies on the bottom of the plate. Daily fungal culture observation with or without sampling suspect dermatophyte colonies and inoculating them on a new culture plate is important to ensure that saprophytes do not overgrow the dermatophytes and potentially cause a false negative culture result.
Additionally, with positive dermatophyte culture results, determining the number of macroscopic colonies gives you information about the severity of infection and, in animals undergoing antifungal treatment, information about the response to therapy.4

Figure 4. Trichophyton mentagrophytes culture often produces a white-to- cream-colored powdery surface. This culture plate has been incubated with inadequate humidity, causing cracking and separation of the culture media on the right side.
Microscopic evaluation of suspect fungal growth is also important since some environmental fungi can mimic dermatophytes in gross colony morphology and in their ability to turn the media red1 and because some strains of Microsporum canis may not produce media color change.6 Microscopic examination can be done in the clinic, or the entire culture plate can be sent to a reference laboratory for fungal identification (usually at a reduced cost compared with fungal culture).
Microscopic identification process
Because the organisms are zoonotic, wear gloves to avoid transmitting dermatophyte spores to your hands. Gently touch a small piece of clear acetate tape to the surface of the fungal colony, and then apply the tape to a glass slide over a drop of blue stain (methylene blue, lactophenol cotton blue, or the blue Diff-Quik solution [basophilic thiazine dye]) (Figure 5). Examine the slide under 100X to 400X magnification to identify the characteristic dermatophyte macroconidia.

Figure 5. To obtain a sample for microscopic fungal identification, touch the tape to the top of the fungal colony and then carefully apply the tape to a slide on top of a drop of blue stain.
In the early stages of growth, only fungal hyphae with no macroconidia may be seen, especially in cases of Trichophyton species infections. Incubate these cultures longer to allow spore development for more reliable identification.
Microscopic dermatophyte characteristics
Microsporum canis spores are large, spindle-shaped, and thick-walled with six or more internal cells (Figure 6) and often have a terminal knob. If M. canis is identified, then other animals in the household should be screened via dermatophyte culture using the toothbrush technique to determine whether they are asymptomatic carriers. All pets with positive culture results should be treated with topical antifungal therapy, with or without systemic treatment. Culture-positive animals should be isolated from culture-negative animals if possible.

Figure 6. Microsporum canis macroconidia and fungal hyphae (Diff-Quik, 400X).
Microsporum gypseum produces large spindle-shaped spores with thin walls, no terminal knob, and six or fewer internal cells (Figure 7).
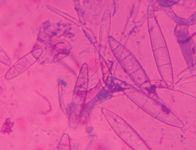
Figure 7. Microsporum gypseum has numerous macroconidia with no terminal knob and thinner walls and fewer internal cells than M. canis has (Diff-Quik, 400X).
Trichophyton mentagrophytes produces long cigar-shaped macroconidia with thin walls (Figure 8). Spiral-shaped hyphae and numerous small grapelike clusters of microconidia are also characteristic of Trichophyton species (Figure 9).1
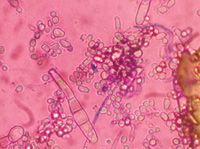
Figure 8. Trichophyton mentagrophytes is characterized by cigar-shaped macroconidia, which may be few in number, and numerous globose microconidia (Diff-Quik, 400X).
Saprophytic fungi will form hyphae and often small spores, but do not form macroconidia.
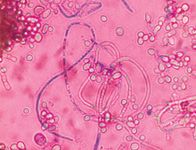
Figure 9. Spiral hyphae are often characteristic of T. mentagrophytes (Diff-Quik, 400X).
In cases in which the fungal species cannot be easily identified in the clinic, submit the dermatophyte culture to a veterinary reference laboratory for fungal identification.
CONCLUSION
Diagnosing dermatophytosis in companion animals can be challenging. However, with appropriate quality control and practice, your in-house dermatophyte cultures will be more successful—and you may even reduce your need to send samples to a reference laboratory.
Nevertheless, if optimal culture media storage and dermatophyte culture incubation conditions, daily observation of fungal colony growth and media color change, and subsequent microscopic identification of suspect fungal organisms are not feasible in your clinic, then submitting samples of surface skin debris and hair (placed in a sterile red top tube) from suspect cases to a veterinary reference laboratory for fungal culture is recommended to avoid misdiagnosis. Even some veterinary dermatologists elect this option to minimize the chance of false negative or false positive dermatophyte culture results.
Kimberly S. Coyner, DVM, DACVD
Dermatology Clinic for Animals of Las Vegas
5231 W. Charleston Blvd.
Las Vegas, NV 89146
REFERENCES
1. Scott DW, Miller WH, Griffin CE. Muller and Kirk's small animal dermatology. 6th ed. Philadelphia, Pa: WB Saunders Co, 2001;121-124.
2. Moriello KA, Newbury S. Recommendations for the management and treatment of dermatophytosis in animal shelters. Vet Clin North Am Small Anim Pract 2006;36:89-114.
3. Hirsh DC, Maclachlan NJ, Walker RL. Veterinary microbiology. 2nd ed. Oxford, U.K.: Wiley-Blackwell, 2004;273-278.
4. Moriello KA. Management of dermatophyte infections in catteries and multiple-cat households. Vet Clin North Am Small Anim Pract 1990;20:1457-1474.
5. Vetlab Supply. DermatoPlate/DermatoPlate-Duo/Dermatoplate S-Duo Culture System product information and instructions. Available at: www.vetlab.com. Accessed May 20, 2010.
6. Moriello KA, Deboer DJ. Fungal flora of the haircoat of cats with and without dermatophytosis. J Med Vet Mycol 1991;29:285-292.