How to simplify management of complex uroliths
Recently a colleague asked us for advice about how to prevent recurrence of a urolith that contained a nucleus of 100 percent calcium oxalate (CaOx) and a distinct outer layer of 95 percent magnesium ammonium phosphate (MAP) and 5 percent calcium phosphate (Image 1 and Figure 2, p. 12S).
Recently a colleague asked us for advice about how to prevent recurrence of a urolith that contained a nucleus of 100 percent calcium oxalate (CaOx) and a distinct outer layer of 95 percent magnesium ammonium phosphate (MAP) and 5 percent calcium phosphate (Image 1 and Figure 2, p. 12S). The urolith was surgically removed from the urinary bladder of a 10-year-old spayed female Yorkshire Terrier. Do you know the name used to classify this complex type of urolith? How would you manage this case?
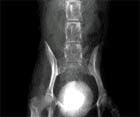
Image 1: Ventrodorsal survey radiograph of the abdomen of a 10-year-old spayed female Yorkshire Terrier. Note the different radiographic density of the compound urocystolith.
What are compound uroliths?
Although some uroliths are composed of only one (100 percent) mineral, most contain a predominant mineral (>70 percent) mixed with lesser quantities of other minerals. If a single mineral does not comprise at least 70 percent of a urolith, and two or more minerals are mixed with each other, it is designated as a mixed urolith.
On occasion different minerals are separated into distinct bands or layers. If the core or center of a urolith is at least 70 percent one mineral type (e.g. CaOx), and is surrounded by one or more layers primarily (>70 percent) of a different mineral (e.g. MAP), is called a compound urolith (Image 1). The Yorkshire described above had a compound urolith.
Compound uroliths comprised approximately 8 percent of the uroliths submitted to the Minnesota Urolith Center in 2002 (Table 1). Compound uroliths form because factors initially promoting precipitation of one type of mineral have been superceded by factors promoting precipitation of a different mineral.

Table 1: Distribution of 1500 Canine Compound Uroliths
For example, antibiotics and urine acidifiers are used to manage infection-induced MAP uroliths. The antibiotics may eradicate or suppress microbial urease, reducing precipitation of MAP. However, acidemia associated with urinary acidifiers may promote hypercallciuria, resulting in a surrounding shell of calcium CaOx or calcium phosphate.
Likewise, we have observed shells of sulfadiazine surrounding some uroliths (e.g., MAP, CaOx) after empirical administration of sulfonamide antimicrobics to patients with signs of lower urinary tract disease.
Some minerals may serve as a template for deposition of other minerals. This phenomenon may explain why CaOx uroliths occasionally have a nidus of silica and vice versa. All uroliths predispose patients to bacterial urinary tract infection (UTI). If UTI by microbes that produce urease persist, there is an increased risk that MAP will precipitate over existing metabolic uroliths (e.g., CaOx, calcium phosphate, urate, silica etc.).
Eliminating compound uroliths
What protocols can be used to eliminate compound uroliths?
Because risk factors that predispose to precipitation of different minerals in compound uroliths are often complex, designing effective medical protocols to manage them can be a unique challenge.
One strategy is to design protocols to dissolve the outer layer first. Serial survey radiographs may be helpful in monitoring the effectiveness of this method. Once the outer layer of different radiographic density disappears, and there is no further reduction in urolith size, medical therapy can be adjusted to dissolve different minerals in the inner layers.
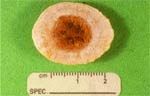
Figure 2: Photograph of the compound urocystolith removed from the dog described in Image 1. The nucleus was composed of calcium oxalate. The shell was composed primarily of magnesium ammonium phosphate.
In some cases, we have reduced the size of compound uroliths by dissolving their outer layers, followed by removing the remaining portion by voiding urohydropropulsion. Symptomatic compound uroliths that are refractory to medical protocols should be removed surgically.
However, in some patients with asymptomatic uroliths, we have chosen a nonsurgical "wait and watch" strategy.
Minimized recurrence
What steps should be followed once compound uroliths have been removed? In the absence of clinical evidence to the contrary, we recommend prevention protocols principally designed to minimize recurrence of minerals that comprised the nucleus, rather than the shell, of compound uroliths. This involves the concept of heterogeneous nucleation.
What is heterogeneous nucleation? Briefly, greater concentrations of lithogenic minerals are required for uroliths to precipitate in the absence of solids in the lumen of the urinary tract (homogenous nucleation) than are required for uroliths to precipitate around a pre-existing solid (e.g., suture material, catheters, and even pre-existing uroliths of a different mineral type).

Figure 3: Photograph of a compound urocystolith removed from an adult male neutered mixed breed dog. The nucleus was composed of silica. The shells were composed of layers of struvite with varying quantities of calcium phosphate.
In the context of compound uroliths, logic suggests that the initial core composed of one mineral type and formed by homogeneous nucleation contributed to the formation of outer layers of a different mineral type formed by heterogeneous nucleation. Therefore, minimizing risk factors for precipitation of minerals found in the core would eliminate heterogeneous nucleation and thus would minimize precipitation of minerals found in the outer layers of the urolith.
Excessive concentration of minerals in urine is a prerequisite for urolith formation. It follows that increased water intake would logically lead to reduction in urine concentration of lithogenic minerals, and thus minimize recurrence of all types of uroliths. In addition to reducing the concentration of lithogenic minerals, formation of large volumes of less concentrated urine decreases the risk of urolithiasis by increasing the frequency of micturition and thus the frequency that crystals would be voided.
To minimize formation of concentrated urine, we recommend feeding high-moisture canned foods. Alternatively, water can be added to dry diets with the goal of achieving a urine specific gravity value of <1.020. Although some specific diuretics may be of value in managing certain types of uroliths, in general we avoid indiscriminate use of diuretics because of their propensity for adverse effects (e.g., dehydration, hypokalemia, hypercalcemia and increased urinary excretion of some lithogenic minerals).
Specific recommendations
Calcium oxalate core: The most common type of compound urolith identified at the Minnesota Urolith Center in 2002 contained a core of CaOx monohydrate or CaOx dihydrate or both (60 percent).
Unlike uroliths predominantly composed of CaOx, which occur more often in males, the majority (81 percent) of compound uroliths with a calcium oxalate core and a MAP shell occurred in female dogs (Image 1 and Figure 2). The paradox in managing patients forming uroliths with CaOx and MAP is that attempts to minimize risk factors for MAP urolith formation (such as reducing urine pH, magnesium and phosphorus) increase the risk for CaOx urolith formation. In this situation, we recommend that emphasis be placed on minimizing recurrence of CaOx uroliths since CaOx uroliths cannot be dissolved medically. In contrast, MAP uroliths that form secondary to infections with urease-producing microbes can often be dissolved by medical protocols. For uroliths containing a core of CaOx surrounded by a shell of infection-induced MAP, it is logical to assume that an initial episode of CaOx uroliths predisposed the patient to infection-induced MAP uroliths. Therefore, preventative management should include efforts to eradicate or control recurrent UTI's.
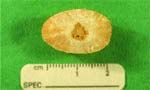
Figure 4: Photograph of a compound urocystolith removed from a 5-year-old spayed female Miniature Schnauzer. The nucleus was composed of ammonium urate. The shell was composed of magnesium ammonium phosphate.
In 18 dogs, a shell of calcium phosphate surrounded a core of CaOx. We hypothesize that excessive calcium excretion was a primary abnormality in these dogs. Since control of urine calcium excretion is emphasized in the management of CaOx uroliths, we use the same principles designed for uroliths composed entirely of CaOx to treat this type of compound urolith. Some compound uroliths contain a core of CaOx and a shell of urate salts. Consumption of diets moderately reduced in protein that promotes formation of alkaline urine commonly recommended to manage CaOx uroliths (such as Prescription Diet Canine u/d-canned; Hill's) are also recommended for prevention of urate uroliths.
Magnesium ammonium phosphate core: 23 percent of compound uroliths contained a core of MAP (Table 1, p. 10S). As typical of infection-induced uroliths, the majority of this type of compound urolith occurred in female dogs (83 percent). Other mineral salts surrounding MAP included calcium oxalate (35 percent), ammonium urate (5 percent), and silica (3 percent). Shells of sulfadiazine surrounded three MAP uroliths. Dogs that formed these stones had a history of symptomatic treatment of lower urinary tract signs with trimethoprim/sulfadiazine.
Calcium phosphate (primarily carbonate apatite) was the most common mineral found in the outer layers surrounding cores of MAP (56 percent). This is predictable because MAP and calcium phosphate uroliths share several common risk factors. For example, the solubility of both salts is reduced in alkaline urine. Also increasing urine phosphate concentration increases the risk of formation of both types of minerals. Precipitation of MAP and calcium phosphate are promoted by urinary tract infections with urease producing microbes that hydrolyze urea into ammonia and carbonate; ammonia is a component of MAP uroliths and carbonate is a component of calcium phosphate (carbonate apatite) uroliths. Why these salts sometimes form distinct layers in some compound uroliths, but become mixed throughout other uroliths without forming distinct layers (mixed uroliths) has not yet been defined.
Fortunately most recommendations to minimize MAP urolith recurrence also minimize formation of calcium phosphate. Treatment with appropriate antimicrobics to eradicate or control of UTI's caused by urease producing microbes is essential. Reducing dietary protein to reduce the urine concentration of urea will minimize the quantity of ammonia generated by microbial urease. In addition, reduction of dietary protein also minimizes renal medullary urea and thus promotes polyuria.
What about diets designed to acidify urine? On one hand, acidification of urine would minimize the quantity of ionic phosphate available to form MAP and calcium phosphate. On the other hand, however, chronic acidification would promote urine calcium excretion and thus increase the risk for formation of uroliths containing calcium. Attempts to acidify urine of dogs with pre-existing MAP uroliths may be one factor that helps to explain why CaOx surrounded 35 percent of uroliths with a core of MAP (see previous section for management of CaOx uroliths with a CaOx core).
Silica core
Compound uroliths with a silica core were primarily retrieved from male dogs (88 percent; Figure 3). The most common mineral associated with silica was CaOx (80 percent). Perhaps one common denominator linking these two minerals is consumption of plant-based foods that contain more silica and oxalic acid that animal-based foods. In addition, one mineral may serve as a template for precipitation for the other.
On the basis of logic, protocols to minimize silica urolith recurrence have been devised. Since diets containing substantial quantities of corn gluten feed or soybean and rice hulls, or both, have been associated with silica uroliths and may also have increased quantities of oxalic acid, we recommend that diets containing substantial quantities of plant proteins be avoided. When CaOx or urate surrounds a core of silica, strategies for prevention of the outer CaOx layer are usually compatible with a reduction in silica urolith formation. When compound uroliths with a core of silica have an outer layer of MAP, urinary tract infection should also be controlled.
Calcium phosphate core
Three percent of compound uroliths contained a core of calcium phosphate. They were surrounded either by shells of CaOx or MAP. We managed these uroliths in a fashion similar to uroliths with cores off CaOx or MAP and outer layers of calcium phosphate.
Ammonium urate core
Only 5 percent of compound uroliths contained a core of ammonium urate. Of those surrounded by a shell of MAP, 70 percent occurred in females (Figure 4). It is logical to assume that the original urate urolith predisposed the patient to UTI with urease-producing microbes, which in turn, promoted formation of MAP. Strategies to prevent recurrence of this type of compound urolith should include control of UTI's in addition to protocols designed to prevent recurrence of urate uroliths.
Of urate uroliths surrounded by a shell of CaOx, 80 percent occurred in males. Consumption of diets moderately reduced in protein that promote formation of alkaline urine commonly recommended to manage CaOx uroliths (such as Prescription Diet Canine u/d-canned; Hill's) are also recommended for prevention of urate uroliths.
Xanthine core
The two dogs with compound uroliths containing a core of xanthine were both being treated with allopurinol to minimize recurrence of urate urolithiasis.
Unfortunately, as the urine concentration of uric acid declines in response to allopurinol, the concentration of xanthine increases. The magnitude of xanthinuria increases in proportion to the quantity of purines in the diet and the dose and frequency of allopurinol administration. Prevention of recurrence encompasses reduction of dietary purines and discontinuing or reducing the dose of allopurinol.
Monitoring
Treatment of compound uroliths often necessitates combining several unrelated treatment regimens.
Therefore, it is essential to monitor the efficacy and safety of therapy. Control of urolith-forming risk factors should result in reduction of urine concentration (e.g., specific gravity) and reduction or elimination of crystalluria. We recommend urinalyses be repeated at appropriate intervals to determine if treatment protocols are associated with desired outcomes.
Because diet modification and drug therapy usually do not eliminate all underlying risk factors, it is unrealistic to expect complete control of recurrent urolithiasis. In our experience, appropriate therapy eliminates urolith recurrence in some dogs, and delays urolith recurrence in others. However, the fact that uroliths recur does not always mean that additional therapy is required. To reduce the need for additional surgery, schedule follow-up evaluations to facilitate detection of urocystoliths when they are small enough to pass easily through the urethral lumen. Small urocystoliths can often be easily removed by voiding urohydropropulsion. If patients are re-evaluated only when they develop clinical signs typical of urolith recurrence, the uroliths often have become too large to pass through the urethra. Regularly scheduled re-examinations also help to promote client compliance with preventative therapy.
Additional information may be obtained at our Web site: www.cvm.-umn.edu Click the link to department and centers to find Minnesota Urolith Center.