How would you manage feline xanthine urocystoliths?
Recently a colleague in private practice asked me for advice about how to treat xanthine bladder stones formed by an 11-month-old, spayed female domestic shorthaired cat.
Recently a colleague in private practice asked me for advice about how to treat xanthine bladder stones formed by an 11-month-old, spayed female domestic shorthaired cat.

Photo 1: Multiple xanthine urocystoliths were removed from a 2-year-old domestic shorthaired cat.
The sources of information she consulted indicated that in dogs the most common cause of xanthine uroliths was an adverse event during treatment with the drug allopurinol. However, she was unable to find information about xanthine uroliths in cats. This cat was not being treated with allopurinol. How would you manage this case?
Epidemiology
Review of samples submitted to the Minnesota Urolith Center revealed that xanthine uroliths were detected in the urinary tracts of 64 cats. None of the cats had been treated with allopurinol. In our series, 60 percent of these cats were males (80 percent of males were neutered and 20 percent were not neutered) and 40 percent were neutered females. The mean age of cats at the time of diagnosis was 2.8 years (range = 4 months to 10 years).
Eight of the 64 cats were less than 1-year old at the time of xanthine urolith detection. Forty-four affected cats were of the domestic short hair breed, 12 were domestic long hair, four were domestic medium hair, one was Siamese, one was designated as British short hair and one was designated as European short hair. The breed of one cat was not identified.
Xanthine uroliths were obtained from the lower urinary tract of 95 percent of these cats, and the upper urinary tract of 5 percent of them.
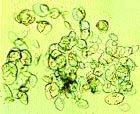
Photo 2: Photomicrograph of xanthine crystals in urine sediment collected from the cat described in Photo 1.
Quantitative analysis revealed that almost all of these stones were pure xanthine. A few contained small quantities of uric acid. Uroliths composed primarily of xanthine were often multiple in number, ranged in size from 1 to 5 mm, and were radiolucent. The physical appearance of xanthine uroliths resembled ammonium urate in that they typically had a smooth surface contour, and were yellow, tan or light brown in color. On cross section, they commonly had numerous concentric laminations that readily separated from each other (Photo 1, p. 14S).
Etiopathogenesis
Xanthine, a normal degradation product of purine metabolism, is converted to uric acid by the enzyme xanthine oxidase (Table 1, p.14S). However, because xanthine is the least soluble of the purines excreted in urine, abnormal quantities of xanthine in urine may result in formation of xanthine uroliths. Acid urine pH, highly concentrated urine, and incomplete and infrequent micturition enhance the risk of xanthine urolith formation.
Naturally occurring (xanthine oxidase deficiency) or drug-induced (allopurinol) impairment of xanthine oxidase ultimately results in hyperxanthinemia and xanthinuria.
We have observed naturally occurring xanthinuria more commonly in cats than dogs. Although the precise underlying abnormality in cats has not been determined, a familial or congenital defect in xanthine oxidase activity is likely. To date, we have not identified a breed predisposition.
In dogs, acquired xanthinuria is a common complication of treatment of urate urolithiasis or Leishmaniasis with allopurinol. Allopurinol is a xanthine oxidase inhibitor, and thus impairs conversion of xanthine to uric acid (Table 1). Consumption of high purine diets increases the risk of xanthinuria in patients treated with allopurinol.

Table 1.
Clinical findings
The initial clinical signs of affected cats were nonspecific (e.g. hematuria, dysuria, pollakiuria and/or urethral obstruction). They were typical of feline lower tract disease due to any cause.
The urine color of some cats with xanthine uroliths was mustard yellow. (Xanthine is the Greek word for "yellow.") Xanthine crystals (which are indistinguishable from amorphous urate or uric acid crystals) were observed in urine samples collected from some cats (Photo 2). Their urine pH was variable, ranging from 6.0 to 8.0. Hematuria was common in cats without concomitant bacterial urinary tract infection. Changes typical of inflammation (pyuria, hematuria and proteinuria) were observed in urine of cats with concomitant urinary tract infections.
The radiodensity of xanthine uroliths is similar to soft tissue. Thus, xanthine uroliths cannot be reliably detected by survey radiography. Double contrast cystography is more sensitive in detecting xanthine urocystoliths than either survey radiography and/or most techniques of ultrasonography. Xanthine uroliths appear radiolucent when surrounded by appropriate concentrations of radiopaque contrast medium. Positive contrast urethrography may be required to detect and localize xanthine uroliths that have passed into the urethral lumen.
Evaluation of hemograms and serum biochemical profiles of a limited number of unobstructed cats revealed no abnormalities. In male cats with urethral obstruction caused by xanthine uroliths, hematological and serum biochemical findings were characteristic of post-renal azotemia. Evaluation of a urine sample of a cat by high-pressure liquid chromatography revealed xanthinuria and a low concentration of uric acid. Further studies are in progress to determine if hyperxanthinemia and hyperxanthinuria associated with reduced serum and urine concentrations of uric acid are a consistent abnormality in cats with xanthine uroliths.
Urolith analysis
Uroliths for analysis may be collected with a tropical fish net during the voiding phase of micturition, by aspiration through a urinary catheter, by voiding urohydropropulsion or by surgery. Uroliths may be submitted to the Minnesota Urolith Center for quantitative analysis. Additional information may be obtained at our Web site: www.cvm.umn.edu. Click the link to department and centers to find Minnesota Urolith Center. Alternatively, FAX your request to (612) 624-0751.
Xanthine cannot be distinguished from ammonium urate and other purine metabolites by polarizing light microscopy. Thus, xanthine may be misidentified if improper methods of analysis are used.
Infrared spectroscopy permits separation of xanthine uroliths from uroliths composed of ammonium urate, sodium urate and uric acid. Caution must be used to avoid use of allopurinol in patients with xanthine uroliths misidentified as ammonium urate uroliths.
Treatment and prevention
Medical protocols that consistently promote dissolution of xanthine uroliths in cats have not yet been developed. At this time, surgery is the most reliable method to remove large active uroliths from the lower urinary tract. However, if urocystoliths are detected while they are still small enough to pass through the urethra, they may be removed by voiding urohydropropulsion.
In our series, uroliths often recurred within three to 12 months following removal from young and middle-age cats. This is not surprising since we are currently unable to correct the underlying metabolic defect. Current recommendations for prevention of recurrence of feline xanthine uroliths encompass reduction in the urine concentration of xanthine and increasing the solubility of xanthine in urine.
This may be accomplished by combinations of dietary modification, diuresis and alkalinization of urine.
Diets that promote excretion of purines in acidic concentrated urine are likely to increase the risk for xanthine urolithiasis in susceptible cats. Pending further studies, we recommend moist renal failure diets (such as canned Prescription Diet Feline k/d, Hill's Pet Nutrition) with the goal of increasing urine volume, minimizing ingestion of purine precursors and minimizing formation of acid urine. If dry diets are fed, add liberal quantities of water to them. Strive to achieve a urine specific gravity value less than 1.025.
Perineal urethrostomies may be considered to minimize recurrent urethral obstruction with uroliths in male cats.