More derm Jeopardy: "These lumps and bumps are for you" (Proceedings)
Cutaneous or subcutaneous nodules are relatively common problems in the dog and cat with assorted etiologies to be considered.
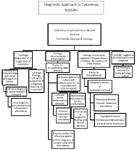
Cutaneous or subcutaneous nodules are relatively common problems in the dog and cat with assorted etiologies to be considered. The diagnostic pursuit is often critical to attaining a definitive diagnosis for optimal clinical management. Systematic approach to the evaluation of these lesions is helpful to achieve the representative data necessary from each case. Signalment and history are important to integrate with the clinical findings to provide information supporting elements of suspicion for differential prioritization prior to the diagnostic approach. Algorithmic approach to the problem may be helpful to avoid oversight of relevant causes. The lesion appearance may be helpful for gross categorization. The presence of well circumscribed lesions versus lesions with less distinct borders should be recognized. The depth of the lesion may also be helpful to develop a differential listing and a diagnostic plan. The recurrence of the lesion in areas where surgery has been performed is consistent with neoplastic disease or infectious causes. Superficial nodules in older dogs are common and often represent benign growths often with characteristic appearances. Response to medications may be helpful to form opinions regarding the categorization of the lesion. Positive response to antibiotics is often suggestive of infectious etiology but does not exclude the possibility of concurrent disease such as mycoses or neoplasia.
The first differentiation of evaluating nodules is to determine or rule out the existence of neoplastic disease in contrast to inflammatory causes or tissue hyperplasia. The acquisition of a minimal data base may include the submission of blood for a complete blood cell count and serum chemistry profile. Thoracic radiographs may be integrated in the initial evaluation if neoplastic disease or systemic (deep) mycoses is suspected. Specimen collection from lesions should initially be examined microscopically. Exudate form open lesions associated with the nodules provide a good source for initial cytology. Needle aspirates should routinely be performed for identifying features of the cytology. Multiple slides should be prepared while collecting specimens from representative lesions. Examination of a specimens stained through a rapid staining procedure such as DiffQuick® (Harleco Corporation) should be performed by the clinician for immediate assessment. If the interpretation by the clinician is inconclusive than the differentiation of neoplasia/hyperplasia versus inflammation may not be possible and decisions may be made on the further diagnostics necessary. Specimens to be sent to an outside laboratory for evaluation should be placed through the first solution of the DiffQuick® for proper fixation before shipping. Protective containers should be used to avoid slide breakage. Further diagnostics should be considered in lieu of waiting for the results of the needle specimen if indicated.
Biopsies are routinely acquired for diagnostic assessment of nodules. Superficial to moderately deep lesions may be biopsied with the use of sedation and local block rather than general anesthesia. The drugs most commonly used by the author is medetomidine (Domitor®, Pfizer Animal Health) for sedation and atipamezole (Antisedan®, Pfizer Animal Health), an alpha 2-adrenergic antagonist, for reversal.. Lidocaine should not contain epinephrine for any skin biopsies. Biopsies may be acquired by use of a skin biopsy punch, scalpel or biopsy needle such as Vim Tru Cut® or Franklin-Silverman biopsy needle. Punch biopsies are most commonly used and are useful for the acquisition of deep specimens from larger nodules/tumors by "double punching". This technique starts with the standard punch specimen removed from the lesion by severing the underlying attachment after applying outward traction on the specimen, then repeating the process through the same biopsy site from which the first specimen was collected. The extraction of the deeper specimen may be more challenging. The use of a small hemostat is often helpful to achieve this. Biopsy punches come in a variety of sizes and from different manufacturers. I recommend maintaining a variety of sizes including 4, 6, 8, & 10 mm.. The biopsy punch preferred by the author is Acu-Punch produced by Acuderm, Inc., Ft. Lauderdale, FL 33309. The use of #15 scapels is often preferred to larger instruments for excising smaller biopsy specimens. Disposable scalpels with fused on handles are available in a variety of forms and sizes (Acuderm, Inc., Ft. Lauderdale, FL 33309). Routine suturing should be attempted if only for possible for cosmetic purposes. Stainless steel (3-0, monofilament) may be used in locations where licking would be anticipated to avoid loosening of the suture. This is also a good method for limiting excessive licking of lesions.
Evaluation of lesions that represent inflammation can often be determined from cytological examination. The most critical evaluation is whether the inflammatory process is infectious or non-infectious. Previous biopsies acquired from the recurrent or persistent lesion or similar lesions failing to identify infectious organisms do not exclude or rule out infectious disease. Atypical mycobacteria, nocardia, deep mycoses, actinomyces and other infectious agents may be difficult to observe in the tissue even with special stains. Cases with exudate and fistulous tracts should be evaluated by cytology with the anticipation of performing special stains such as Gram's. Culture submission of swab specimens obtained from open wounds often have a multitude of secondary opportunistic organisms and are not valuable for assessment of the primary pathogen associated with the lesion(s). Submission of aseptically acquired tissue specimens, transported in specialized transport media (Port-A-Cul®, Becton, Dickinson and Co., Sparks, MD) is an essential part of the diagnostic process that should be performed on inflammatory lesions to assist in the differentiation of infectious versus non-infectious causes. Port-A-Cul® is available through Fischer Scientific. Concurrent submission of specimens fixed in formalin for histopathological examination is necessary to provide adjunctive diagnostic yield for complete evaluation. Both the microbiology laboratory and the histopathology laboratory should have a complete history and listing of suspected diseases or organisms to perform the proper procedures. Requests are typically necessary for fungal culture in addition to aerobic bacterial culture. Susceptability testing should always be requested where possible. Speicial culture media is required for optimal growth of mycobacteria and should be emphasized where applicable. Specimens from multiple lesions should be submitted. Normally 3-5 specimens are used to provide adequate representation for assessment. Deeper and larger lesions should be biopsied under traditional surgical procedures, using general anesthesia, with an aseptic surgical scrub and preparation performed if culture is to be pursued. Laboratory findings (culture & histopath) failing to identify infectious agents DOES NOT exclude the possibility of infectious etiology. Before making a definitive diagnosis and pursuing a therapeutic plan, overall evaluation of diagnostic data with relationship to breed and age of the animal, history, habitat, animal use, potential exposure to infectious agents, and other historical/clinical features are important. Repeat biopsies may be indicated based on the preliminary biopsy results. The worst situation is placing an animal with an infectious disease on high dose glucocorticoid therapy resulting in disseminated infection. Be sure to use reputable diagnostic laboratories that are familiar with the procedures and diseases necessary to achieve a diagnosis. A definitive diagnosis is not always possible. Treating for infection prior to the use of immunosuppressive glucocorticoids is preferable than the reverse approach. Remember that any inflammatory condition will "improve" with anti-inflammatory drugs whether an infectious cause or a sterile inflammatory process. Always err on the side of caution. The following flow chart may be helpful to plan a course for diagnostic testing and follow-up. Inflammatory nodules are the most frustrating.