Nutritional support for hospitalized patients
Nutritional needs often play a secondary role to medical and surgical intervention. Critically ill veterinary patients are at high risk for malnutrition because of physical impediments, as well as physiologic and metabolic abnormalities. Protein and/or calorie malnutrition results in decreased immune competence, decreased tissue synthesis, increased protein degradation (especially that of the lymphatic system), altered drug metabolism and is known to increase morbidity and mortality in human patients. Although veterinary studies are lacking, it is generally accepted that early enteral nutrition decreases complications from malnutrition.
Nutritional needs often play a secondary role to medical and surgical intervention. Critically ill veterinary patients are at high risk for malnutrition because of physical impediments, as well as physiologic and metabolic abnormalities. Protein and/or calorie malnutrition results in decreased immune competence, decreased tissue synthesis, increased protein degradation (especially that of the lymphatic system), altered drug metabolism and is known to increase morbidity and mortality in human patients. Although veterinary studies are lacking, it is generally accepted that early enteral nutrition decreases complications from malnutrition.

Cat with esophagostomy tube in place.
Collecting diet history information from owners is important in directing the feeding plan of any hospitalized animal. The duration of acceptable starvation may be different for a dog that normally eats a complete and balanced commercial dog food as opposed to one that has been eating a home-prepared diet with suboptimal intake of essential fatty acids, vitamins and minerals. Additionally, many cats develop fixed food preferences that can pose a feeding challenge in a hospital setting when changes to food characteristics, such as aroma and texture, may cause them to refuse food even in the absence of a significant disease state.
A 2001 survey of four large referral veterinary hospitals in the United States evaluated nutritional support and feeding orders for 276 dogs that spent a cumulative 821 days (dog days) in the hospital (Remillard, et al. 2001). Of those, 601 dog days were spent in negative energy balance and the reasons cited were poorly written orders (22 percent), orders to withhold food (34 percent) and animals' refusal to eat (44 percent).
Patient selection
Indications for nutritional support include any hyporexic animal (i.e., those with chronic poor dietary intake), animals that have lost more than 10 percent of body weight, shown anorexia or expected anorexia for more than five days, animals on home-prepared diets (i.e., chronic suboptimal intake of essential nutrients), animals with expected ongoing nutrient loss, animals with high energy demand or hypoalbuminemic animals. The appropriate patient is hemodynamically stable and the benefit of nutritional support outweighs the risk of potential aspiration. Any patient that is vomiting, regurgitating or unable to protect its airway is not a candidate for enteral nutrition and parenteral nutrition should be sought.
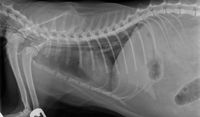
Radiographic assessment of esophagostomy tube placement.
Feeding route
When choosing the appropriate feeding route for nutritional intervention, the primary goal should be to bypass the affected area. Consideration should be given to available route of nutrient delivery, anticipated duration of enteral support, impact of tube size on diet selection, signalment, disease state, condition of the gastrointestinal system, necessary nutrient modifications and overall prognosis. It is important to consider the affect of drug therapy and therapeutic interventions on gastrointestinal motility and, if indicated, consider using promotility agents. All blindly placed feeding tubes should be evaluated for proper placement with two view radiographs and/or capnography.
Nasoesophageal/nasogastric: Selected for short-term (less than 10 days) assisted nutritional support in animals with normal nasal, pharyngeal, esophageal and gastric function. Little to no anesthesia is required for tube placement, making it a relatively quick and inexpensive way to provide short-term nutritional support in a clinic or hospital setting.
Due to traversing of the nasal passages, narrow-diameter feeding tubes (5-8 French) are required. Blended canned food slurries would readily clog these types of tubes, necessitating the use of a liquid enteral solution. Liquid enteral solutions are best delivered as a constant rate infusion to minimize risk of regurgitation and aspiration; the nutrient profiles may not be suitable for all animals and disease states.
Nasogastric tubes allow for suctioning of the stomach, but are associated with an increased incidence of esophageal reflux. The placement of nasaljejunal feeding tubes with the assistance of fluoroscopy has been recently described. Epistaxis, facial irritation and premature tube removal are common complications. Clinical experience is that some patients will not eat with a nasal tube in place. This tube type is contraindicated in animals that are obtunded, unable to maintain sternal recumbency or have weak to absent gag reflexes.
Esophagostomy: These more permanent feeding tubes can be used to facilitate nutritional support in the hospital and then maintained at home for weeks to months. General anesthesia is recommended, but these tubes can be placed with heavy sedation or light anesthesia. Placement is relatively quick and easy in a general veterinary clinic setting and the larger-diameter tube (12-14 Fr for cats and dogs less than 15 kg, 14-18 Fr for dogs larger than 15 kg) allows for the use of most commercial canned diets blended with water. There is minimal risk of life-threatening complications if these tubes are inadvertently removed within the first two weeks, and these tubes are also well tolerated by most animals. Complications include periostomal skin infections, tube dislodgement and tube clogging. Major limitations include inability to monitor gastric residual volume, anesthesia requirement and the fact that the dilution required for many commercial diets may result in a food volume that exceeds tolerance of a given animal.
Gastrostomy: Whether percutaneous endoscopic gastrostomy (PEG), percutaneous blind-placement or surgically placed, gastrostomy-tube placement allows for longer-term nutritional support (months to year) of animals requiring assisted nutritional support. This is the recommended feeding tube in animals with esophageal disorders (esophagitis, megaesophagus or esophageal strictures). Placement of these tubes allows for monitoring residual stomach volumes and the larger diameter (18-24 Fr) allows for a wider range of food selections.
These types of tubes can be placed in a general clinic or hospital setting, but may require a longer anesthetic and procedure time if placed surgically. Animals with gastrostomy tubes are at risk for peritonitis and life-threatening complications if this tube is removed within the first two weeks before the stoma site is healed. Additional complications with blind placement include risk of splenic entrapment and incorrect positioning.
Jejunal feeding and jejunostomy: This assisted-feeding method is indicated in animals that are unable to tolerate gastric feeding but have normal jejunal, ileal and colonoic function. Placement of jejunostomy tubes may be indicated in animals with gastric outflow or proximal small-intestine obstructions and severe pancreatitis. These tubes are most commonly placed surgically (jejunostomy), but newer percutaneous endoscopic gastrojejunal tube (J-G) and fluroscopically guided nasaljejunal tube techniques have been evaluated in dogs and cats and may play a role in the management of hospitalized animals in the future.
Jejunostomy tubes must be placed under general anesthesia and are again limited in diameter (5-8 Fr). Because nutrients delivered into the jejunum have bypassed the major steps in digestion that occur in the stomach and duodenum, liquid elemental diets are preferred with this feeding type and should be delivered as a constant rate infusion over 12 to 16 hours to prevent complications, such as abdominal cramping and diarrhea.
Surgical placement of jejunostomy tubes also carries the risks of tube displacement and subsequent peritonitis. Jejunal feeding through a J-G or nasaljejunal tube eliminates the risk of leakage through a jejunostomy site. These tubes can be removed or "backed out" once pancreatitis or the intestinal disease has resolved and still allow for further enteral nutrition without an additional procedure. These feeding tubes are best utilized in a veterinary hospital where continued monitoring and care can be provided.
Determination of energy needs
The recommendation for energy intake in hospitalized animals has shifted within the last decade. Energy requirements for critically ill animals are largely based on meeting calculated resting energy requirements (RER) and adjusting up or down as needed. The previous use of illness factors (calculated RER multiplied by 1.2 to 1.5) added to the daily feeding recommendation of hospitalized animals has fallen out of favor. In animals with voluntary food consumption, this is more a food-waste issue, but with assisted enteral feeding intolerance to larger food volumes can lead to regurgitation, abdominal cramping, vomiting, diarrhea and potential aspiration of vomitus or regurgitated material.
The two indirect formulas for calculating RER for dogs and cats are allometric (70* BWkg0.75) and linear ([30* BWkg] + 70). The linear formula will overestimate RER for animals less than 2 kg, and as such the allometic formula is preferred for all animals. Initial feeding should deliver 25 percent of the animal's RER over the first day and be increased gradually until RER is reached in two to four days. Prior to each feeding, the tube should be aspirated with an empty syringe to monitor for residual food volume, if applicable. Food should be warmed to room temperature and fed slowly to prevent vomiting. Warm water should be used to flush the tube before and after feeding and after medication administration to prevent clogging. The animal's temperature, respiratory rate, respiratory effort, lung sounds and development of a cough should be monitored closely for any airway complications.
Some disease states may result in hypermetabolism and the amount of energy delivered will need to be increased to accommodate increased losses, such as animals with protein-losing diseases, persistent diarrhea or diabetes mellitus. Like any medical treatment, nutritional plans should be dynamic: Start with a goal to reach calculated RER and adjust the calories delivered each day based on body-weight changes, physical-exam finding and known or expected ongoing losses.
Melissa Marshall, DVM, Dipl. ACVECC, received her veterinary degree from Tufts University in 1999. She completed a rotating internship in small-animal medicine and surgery at Animal Specialty Group and her residency in emergency and critical care at Angell Memorial Animal Hospital. She joined Red Bank Veterinary Hospital in 2005.
Lisa P. Weeth, DVM, Dipl. ACVN, received her veterinary degree from the University of California, Davis in 2002. She worked in private practice for several years prior to returning to the University of California to complete a residency in clinical nutrition. She joined Red Bank Veterinary Hospital in 2007.