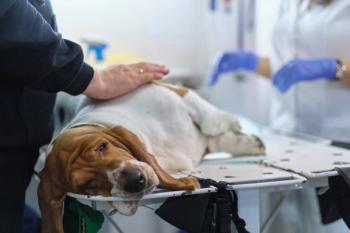
Ohio State Veterinary Medical Center takes part in nationwide canine cancer study
Recruitment for dogs with lymphoma is underway.
The Ohio State University's Veterinary Medical Center (VMC) is currently participating in a nationwide clinical trial to evaluate the efficacy and safety of three novel chemotherapy agents in dogs with lymphoma, according to a university news release. The study, sponsored by the National Cancer Institute, is the first of its kind in evaluating the use of indenoisoquinolines in dogs.
In order to participate in the study, dogs should have a histologically confirmed diagnosis of lymphoma and present with at least one lymph node larger than three centimeters. Dogs should otherwise be in good health, as determined by physical examination and recent blood work results. Before beginning the trial, participating dogs must have a two-week washout period from previous chemotherapy or radiation treatment and be free of corticosteroids or L-asparaginase for at least seven days. Additionally, upon enrollment, dogs will have tumor fine needle aspirate and biopsy, bone marrow aspirate and blood collection performed prior to starting treatment. Both newly diagnosed dogs and those with recurrent or relapsing disease are eligible to participate.
The trial is divided into two phases: a dose escalation phase to determine safety and a validation phase for biological assay development. Dogs will receive daily intravenous administration of an indenoisoquinoline for five consecutive days, with 24-hour serial pharmacokinetic collections on days one and five. Additional tumor aspirates, biopsies and bone marrow aspirates will be collected throughout the 29-day study duration to monitor treatment response.
Participating clients will receive a $1,000 account credit for future veterinary care for their pet at the VMC. Information about the study can be obtained by e-mailing
Newsletter
From exam room tips to practice management insights, get trusted veterinary news delivered straight to your inbox—subscribe to dvm360.