Pulmonary sampling (Proceedings)
Definitive diagnosis of pulmonary disease remains elusive at times. Cytological or histopathological samples are useful to help better classify the underlying cause as well as determine both prognosis and treatment course. Thus, it is prudent for the criticalist to have a strong grasp of the various techniques and options available for sampling.
Definitive diagnosis of pulmonary disease remains elusive at times. Cytological or histopathological samples are useful to help better classify the underlying cause as well as determine both prognosis and treatment course. Thus, it is prudent for the criticalist to have a strong grasp of the various techniques and options available for sampling. Additionally, as many patients with cardiopulmonary disease are relatively unstable, it is wise to recognize the potential risk and benefits of the testing.
The goal of the presentation is to describe the techniques as well as the potential benefits and risks of available methods to assess lung pathology. Available techniques include oral examination with biopsy, bronchoscopy, bronchoalveolar lavage, transtracheal aspirate, transoral tracheal aspirate, fine needle lung aspirate (with or without ultrasound guidance), and thoracocentesis with evaluation of cytological characteristics of the pleural effusion.
As an overview, pulmonary disease is classically localized to upper airway, tracheal/bronchial (lower airway), pulmonary parenchyma and pleural space. Differential diagnoses will vary based upon patient signalment and history. Some patients may require extensive evaluations, while others do not require further evaluation short of a complete history and physical examination. For example, a young dog with pulmonary infiltrates in a pattern consistent with pulmonary contusion after having been hit by a car requires no further diagnostic tests. Conversely, an older dog with a recurrent bout of cough and pulmonary infiltrate may require extensive testing.
Larynx
Common laryngeal diseases include laryngitis (from excessive barking or tracheobronchitis) or laryngeal masses. Laryngeal masses are more common in older patients, particularly cats. Clinical signs of laryngeal masses include slowly progressive loud or stridorous breathing. Some animals have a apparent response associated with prior therapy associated with antibiotics or glucocorticoids. Neoplasia, specifically squamous cell carcinoma is the most common. A granulamatous proliferative (albeit non-neoplastic) condition has also been described in cats.
Biopsy samples are required to definitively identify neoplasia as well as to provide major prognostic information. Two points are of specific importance when considering a laryngeal biopsy. The first point is that all clinically significant obstruction laryngeal disease is not a quick "cure. This is important from the perspective of the client as in contrast with some other disease processes, the presence of laryngeal mass carries a very guarded prognosis. The second major point is that due to the often gradual progression of infiltrative disease, there is often a very tiny remaining airway lumen at the time of oral examination. Any loss of active airway dilating activity may result in complete airway occlusion. Thus, the clinician should be prepared for an emergency tracheostomy with readily available support staff and supplies. Additionally, even in the airway is likely considered adequate to recover the pet from sedation; airway swelling subsequent to a biopsy may result in occlusion. Discussion of a temporary tracheostomy tube should be cleared with the pet's family prior to undertaking an oral examination.
A laryngeal biopsy may be obtained with endoscopy biopsy instruments, long-handled scissor (Metzenbaums) or a biopsy cup forcep. My preference is to use a large cup forcep with the goal to also debulk a major portion of the mass.
Lower airway disease
Common lower airway diseases include feline lower airway disease ("asthma"), eosinophilic pneumonitis and chronic bronchitis. Cytological examination with bacterial culture and sensitivity testing is warranted in most cases. Options for collection of cytological samples include transoral tracheal wash, transtracheal wash (aspirate), and bronchoscopy with bronchoalveolar lavage. Bronchoscopy is considered the gold standard for evaluation of the lower airways and collection of cytological samples. However, due to financial constraints or concerns regarding anesthesia, other options are often pursued.
A transoral approach for collection of samples is warranted in all cats and small dogs. Many clinicians prefer to perform a transoral approach in all sized pets. Sample collection should not be pursed if subsequent sedation is considered risky (eg. Inadequate supplies/training/support staff or marked respiratory distress in the patient). Necessary supplies include supplemental oxygen, propofol, a laryngoscope, sterile endotracheal tube, sterile specimen cup, and sterile long red rubber urinary catheter (5 or 8 Fr) and sterile 3 aliquots of saline (3-5 ml for small pet, 5-10 for medium dog, and 10-15 for large dog). Saline is used to avoid cell lysis. The patient should be pre-oxygenated for 2-5 minutes and then anesthesia induced with propofol IV to effect. The sterile endotracheal tube should be placed. Do NOT use any lubricant on the tube, although it is acceptable to use a smaller tube to facilitate intubation. The cuff may be gently inflated if desired and supplemental flow-by oxygen may be administered. Allow the pet to wake up a bit, so that a cough reflex is restored. I will often take initial advantage of the sedation in order to perform an oral examination. Monitoring with a pulse ox and EKG is wise. Pass the catheter through the endotracheal tube and then infuse the saline. Simultaneously, aspirate back on the syringe and move the head position to allow fluid to drain (expectorate) into the sterile cup. The collected fluid should be placed into an EDTA tube as well as a red top or culturette. Some clinicians like to use a self-contained suction system to actively aspirate samples. However, my anxiety in a teaching hospital is that it creates the impression that elaborate supplies are needed to perform a tracheal wash and thus may frighten students from performing this procedure in the future.
Fluid retrieved should be evaluated cytologically following a concentration technique (eg. Cytospin) and also cultured for aerobic bacteria. There is some evidence that Mycoplasma spp play a role in respiratory diseases of cats and dogs. Thus as indicted clinically, culture or PCR for these organisms would be warranted.
Transtracheal aspirate (TTA) was particularly popular in the days before the ready availability of propofol. TTA are somewhat technically challenging, particularly in big or uncooperative dogs. Supplies required to performed a TTA include sterile gloves, prep solution, local anesthesia, a through the needle catheter (eg. Intracath) and aliquots of saline. A TTA is performed by clipping and prepping the site over the trachea or cricothyroid membrane (larynx). Either is fine, although in larger dogs the lower you go in the airways the better samples you get. The site of intended puncture is identified and infiltrated with local anesthesia. The catheter is inserted into the airway and advanced distally. Coughing should ensue. If the catheter is adequately placed, air should be easily aspirated back though the catheter. The saline aliquots are similarly infused though the catheter and retrieved. Subcutaneous emphysema is a potential complication.
Bronchoscopy with collection of samples is the best way to evaluate the lower airways. Like any technique, practice and experience will improve your assessment and diagnostic yield (ie- do lots of bronchoscopy!). In contrast with GI endoscopy, at times the airways may be confusing. McKiernan and Amis developed the classic "road map" of the canine airways. This should be posted at a readily accessible area near the bronchoscopy suite.
Pulmonary parenchyma/interstitium
The pulmonary parenchymal changes may include either alveolar or interstitial changes. Alveolar changes are most commonly either edema or pneumonia. Cardiogenic pulmonary edema is typically easy to recognize as cardiomegaly, murmur and left atrial enlargement are common. Sampling of pulmonary edema per se rarely is required. However, if an animal is intubated for support of respiratory failure and edema is present, it is appropriate to collect a sample for evaluation of cytology and protein content. Cardiogenic pulmonary edema (due to increased hydrostatic pressure) will have a very low total protein in relation to serum protein (Usually < 0.4) while non-cardiogenic pulmonary edema (due to vascular permeability) will have a protein content that approximates serum protein. Samples from animals with suspected pneumonia should be collected via either a tracheal wash or bronchoscopy.
For animals with primarily interstitial lung disease, it is prudent to recognize that a tracheal wash will be fairly low yield (common "mild inflammation" no growth on culture). The best options for sampling the lung interstitium are either a fine needle aspirate or preferentially a lung biopsy (either via open lung biopsy or via thoracoscopic examination). A fine needle lung aspirate may be performed either blindly or with ultrasound (or CT guidance). FNA is commonly very helpful. In animals with diffuse disease, pneumothorax is a real possibility following aspiration, so pets should be carefully observed for at least several hours following the procedure.
Pleural effusion
Pleural effusion is frequently sampled for both diagnostic and therapeutic ends. Cytological examination remains an essential aspect of evaluation of the pet with pleural effusion. Markers in pleural effusion are commonly evaluated in people but have rarely been looked at in animals. It is wise to recall that with malignant pleural effusions, neoplastic cells may be relatively hard to identify and large quantities of fluid may need to be evaluated to confirm the diagnosis.
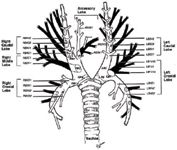
Diagram of the endobronchial anatomy in normal dogs. Originally published by Amis, T and McKiernan, BC.
References
Amis T and McKiernan BC. Systemic identification of endobronchial anatomy during bronchoscopy in the dog. Am J Vet Research 1986, 47: 2649-2657.
Rha J and Mahony O. Bronchoscopy in small animal medicine: indications, instrumentation, and techniques. Clin Tech Small Anim Pract 1999 14: 207-212.
McCullough S and Brinson J. Collection and interpretation of respiratory cytology. Clin Tech Small Anim Pract. 1999 Nov;14(4):220-6.
Norris CR et al. Use of keyhole lung biopsy for diagnosis of interstitial lung diseases in dogs and cats: 13 cases (1998-2001). J Am Vet Med Assoc. 2002 Nov 15;221(10):1453-9.
DeBarry JN et al. Correlation between fine-needle aspiration cytopathology and histopathology of the lung in dogs and cats. J Am Anim Hosp Assoc. 2002 Jul-Aug;38(4):327-36.
Bailiff NL et al. Clinical signs, clinicopathological findings, etiology, and outcome associated with hemoptysis in dogs: 36 cases (1990-1999). J Am Anim Hosp Assoc. 2002 Mar-Apr;38(2):125-33.
Norris CR et al. Comparison of results of thoracic radiography, cytologic evaluation of bronchoalveolar lavage fluid, and histologic evaluation of lung specimens in dogs with respiratory tract disease: 16 cases (1996-2000). J Am Vet Med Assoc. 2001 May 1;218(9):1456-61.
Miedouge M et al.Evaluation of seven tumour markers in pleural fluid for the diagnosis of malignant effusions. Br J Cancer. 1999 Nov;81(6):1059-65.
A guide for assessing respiratory emergencies
November 15th 2024Mariana Pardo, BVSc, MV, DACVECC, provided an overview on breathing patterns, respiratory sounds, lung auscultation; and what these different sounds, patterns, and signs may mean—and more—in her lecture at the 2024 NY Vet Show
Read More