Secondary hemostasis (Proceedings)
The goal of secondary hemostasis is to stabilize the platelet aggregate by weaving a meshwork of fibrin to cement the thrombus.
Normal hemostasis occurs in three main stages. Primary hemostasis occurs when platelets attach to a damaged or disrupted area of the endothelium. This adhesion allows the platelets to undergo a shape change and then aggregate together. Once adhered to each other a temporary platelet plug is created. However, this platelet plug is not stable for long if the secondary hemostatic forces do not strengthen and reinforce the plug with a crosslinked fibrin mesh. The final stage of coagulation involves fibrinolysis. In this stage, plasminogen is activated to plasmin, which then breaks down the fibrin and removes the clot.
Secondary Hemostasis
The goal of secondary hemostasis is to stabilize the platelet aggregate by weaving a meshwork of fibrin to cement the thrombus. In vitro, the classic coagulation cascade maintains this role. The intrinsic pathway (Factors VIII, IX, XI, XII) and the extrinsic pathway (Factor VII and Tissue Factor) join to activate Factor X and the common pathway. The end result is the formation of thrombin, which cleaves fibrinogen to fibrin and thus the clot can be stabilized.
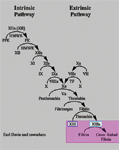
In vivo, the cell based model of hemostasis prevails. The cell based model of hemostasis has three stages: initiation, amplification and propagation. Initiation of coagulation is triggered by tissue factor (TF), a cellular 'receptor' for activated coagulation factor VIIa (FVIIa) that circulates in plasma at a very low concentration. TF is, like collagen, present in the subendothelial matrix and is thus exposed to blood after a vascular injury. The complex formed between TF and FVIIa will then activate FIX and FX, to FIXa and FXa, respectively. Although FXa is a poor activator of prothrombin, enough thrombin is generated to initiate a series of new activation steps. Thrombin converts a small amount of fibrinogen into fibrin strands that start to 'precipitate' and cause the primary platelet plug to stabilize. The initial phase of the coagulation system generates only a small amount of thrombin.
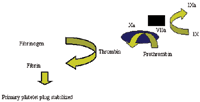
Thrombin generated during the initiation phase will then activate additional platelets and the coagulation factors V, VIII and XI. Activation of FV and FVIII is a very important step, as it will result in a huge amplification of thrombin generation. In order to localize thrombin generation to the site of injury, FIXa is bound to phospholipids which are exposed on the membrane of activated platelets in the primary platelet plug. FIXa on the platelet membrane will then bind to its cofactor, FVIIIa, and form the tenase complex which is a potent activator of FX. Activated FX will also, like FIXa, bind to the platelet membrane and together with its cofactor, FVa, form the prothrombinase complex which is a much more potent activator of prothrombin than FXa alone. More than 90% of the thrombin that is formed during coagulation is generated via the action of the tenase and prothrombinase complexes. Thus these complexes allow the continued production of thrombin (ie propagation).
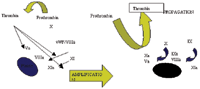
Once thrombin converts fibrinogen to soluble fibrin monomers, cross-linking and stabilization of these monomers is mediated by Factor XIII (which is concurrently being activated by thrombin). Thrombin activated fibrinolytic inhibitor (TAFI) down regulates fibrinolysis, thus helping to maintain the clot.
Physiologic and Pharmacologic Inhibitors of Secondary Hemostasis
Fibrin clot generation must be restricted to the site of vessel damage. Physiologic and pharmacologic inhibitors act to localize and dampen the procoagulant reactions of the clotting cascade.
Tissue Factor Pathway Inhibitor (TFPI)
It is synthesized and expressed by the endothelium. A small amount is stored in platelets and secreted on activation and degranulation. The majority of circulating TFPI is bound to lipoproteins. It inhibits TF-VIIa activity in two ways. In combination with heparin it has a mild inhibitory activity toward factor Xa. Secondly the complexing of TFPI with factor Xa completely inhibits the TF-VIIa activation of factors IX and X. The overall effect is the inhibition of the initiation phase of the coagulation system.
Antithrombin
It is produced by the liver, endothelium and megakaryocytes. For antithrombin to exert its action it must bind with heparin sulfate. Antithrombin inhibits factors IX, X and thrombin thus preventing it from converting fibrinogen to fibrin. Antithrombin binds to selected forms of glycosaminoglycans found on endothelial membranes resulting in an increase in prostacyclin synthesis. This limits interactions between endothelial cells and neutrophil, reduces platelet aggregation, and decreases pro-inflammatory cytokine production. This effect is abolished by exogenous heparin administration.
Protein C/Protein S Pathway
Once thrombin is formed it can either cause fibrin formation and platelet and endothelial cell activation, or it can bind to thrombomodulin (TM), where it rapidly activates protein C. Thrombomodulin converts thrombin from a procoagulant to an anticoagulant protease. The binding of these two compounds converts protein C to its active form APC via a receptor, called endothelial protein c receptor (EPCR), on the endothelial cell. Activated protein C down regulates thrombin generation by greatly accelerating the proteolytic destruction of the major cofactors of the coagulation cascade, factors Va and VIIIa. APC also promotes fibrinolysis and inhibits inflammation and thrombosis. Protein S is synthesized and secreted by endothelial cells, binding to the surface of endothelial cells, where it forms a macromolecular complex with activated protein C. This binding accelerates the rate of inactivation of factors Va and VIIIa. Protein S may also directly inhibit prothrombinase in the absence of activated protein C through direct interactions with factor Xa.
Anticoagulants
Unfractionated heparin (UFH)
Commercial UFH is obtained from either bovine lung or porcine intestinal mucosa and consists of a heterogeneous mixture of polysaccharides (glycosaminoglycans) with molecular weights ranging from 4000 to 30,000 daltons (d), with a mean molecular weight of approximately 15,000 d (approximately 45 saccharide units). Once heparin is present in the circulation (either exogenously or endogenously (via endothelial disturbance) it binds to antithrombin (AT). The heparin-AT complex inactivates a number of coagulation enzymes, including thrombin, factors Xa, IXa, XIa, and XIIa. Of these, thrombin and factor Xa are most responsive to inhibition.
Low Molecular Weight Heparin (LMWH)
LMWH is derived from the enzymatic or chemical cleavage of UFH to produce a mixture of low-molecular-weight glycosaminoglycan molecules with a mean molecular weight of approximately 5000 d (approximately 15 saccharide units). Binding of the pentasaccharide to antithrombin causes a conformational change in antithrombin that accelerates its interaction with thrombin and activated factor X (factor Xa) by about 1000 times.The chief difference between unfractionated heparin and low-molecular-weight heparins is in their relative inhibitory activity against factor Xa and thrombin.Any pentasaccharide-containing heparin chain can inhibit the action of factor Xa simply by binding to antithrombin and causing a conformational change In contrast, to inactivate thrombin, heparin must bind to both antithrombin and thrombin, thereby forming a ternary complex. This complex can be formed only by pentasaccharide-containing heparin chains composed of at least 18 saccharide units. Whereas most of the chains of unfractionated heparin are at least 18 saccharide units long, fewer than half of those of low-molecular-weight heparins are of sufficient length to bind to both antithrombin and thrombin. Consequently, unlike unfractionated heparin, which has equivalent activity against factor Xa and thrombin, low-molecular-weight heparins have greater activity against factor Xa.
UFHand LMWH also induces secretion of TFPI by vascular endothelial cells thus reducing the procoagulant activity of tissue factor VIIa complex. This effect could contribute to the antithrombotic action of heparin and LMWH.
Coumarin (Warfarin)
Coumarin exerts its effects by inhibiting the enzyme vitamin K epoxide reductase. Vitamin KH2 then becomes depleted, preventing carboxylation of the inactive prozymogens (ie factors 2, 7,9,10 and protein C and S). The blockade results in incomplete molecules that are biologically inactive (unable to undergo calcium-mediated binding of prothrombin to platelet phospholipids) and are also known as PIVKA (proteins induced in vitamin K absence).
Hirudin
It is derived from the salivary glands of medicinal leeches (Hirudo medicinalis). Hirudin inhibits both free and clot bound thrombin.
Fibrinolysis
Fibrinolysis is the enzymatic degradation of fibrin. Plasmin is the serine protease that is responsible for this degradation and is derived from its inactive precursor plasminogen. The activation of plasminogen is caused by tissue type plasminogen activator (tPA) and urokinase type plasminogen activator (uPA). tPA is derived from endothelial cells and is released in its active form. It primarily activates plasminogen bound to the fibrin clot. Urokinase type plasminogen activator is secreted by cells in the kidney and is secreted in its inactive form (activated by kallikrein). Once plasmin is generated it degrades fibrin. The degradation of fibrin produces fibrin degradation products (FDPs). FDP's can bind thrombin and prevent further fibrin formation and therefore act as local anticoagulants. Inhibitors of fibrinolysis will be discussed during presentation space restriction for proceedings.
References
Hopper K, Bateman S. An updated view of hemostasis: mechanism of hemostatic dysfunction associated with sepsis. JVECC 15(2) 2005, pp 83-91.
Roberts HR, Monroe DM, Escobar. Current Concepts in Hemostasis. Anesthesiology 100 (3) 2004 pp 722-30.
Podcast CE: A Surgeon’s Perspective on Current Trends for the Management of Osteoarthritis, Part 1
May 17th 2024David L. Dycus, DVM, MS, CCRP, DACVS joins Adam Christman, DVM, MBA, to discuss a proactive approach to the diagnosis of osteoarthritis and the best tools for general practice.
Listen