Shock and resuscitation: Be a shock buster (Proceedings)
Small animals in crisis will present for a myriad of reasons: vomiting, diarrhea, bloat, urinary obstruction, dystocia, trauma...the list is endless. A rapid assessment must determine whether the animal has decompensated or is likely to decompensate. Decompensation will be directly related to Airway, Breathing and/or Circulatory failure.
"The single most important factor in successful resuscitation from shock is time: rapid expeditious therapy in the early stages may lead to good results, but adequate therapy that is delayed may be ineffective." (Shoemaker, W.C)
Small animals in crisis will present for a myriad of reasons: vomiting, diarrhea, bloat, urinary obstruction, dystocia, trauma...the list is endless. A rapid assessment must determine whether the animal has decompensated or is likely to decompensate. Decompensation will be directly related to Airway, Breathing and/or Circulatory failure.
Shock is a phenomenon of ineffective circulating volume. Significant loss of intravascular volume, or hypovolemia, can result from many causes, to include: trauma, loss of plasma water during vomiting and diarrhea, extreme venodilation from systemic inflammation, and significant hemorrhage. A positive outcome is optimized by rapid and aggressive fluid resuscitation, with hemostasis employed as required. The ability to create an effective fluid resuscitation plan depends on understanding the pathophysiology of shock and the different body fluid compartments and the dynamics of fluid movement and distribution between fluid compartments.
Body fluid compartments and fluid dynamics
There are 3 major fluid compartments; intravascular, interstitial and intracellular. Fluid movement from the intravascular to interstitial and intracellular compartments occurs at the capillary. A capillary "membrane, consisting of capillary endothelial cells and the subendothelial cell matrix separates the capillary intravascular space from the interstitial fluid compartment. This capillary "membrane" is freely permeable to water and small molecular weight particles such as ions, glucose, acetate, lactate, gluconate, and bicarbonate. Gases such as oxygen and carbon dioxide diffuse freely through the capillary endothelial cell to enter or exit the intravascular compartment.
The interstitial compartment is that space between the capillaries and the cells. Fluids support the matrix and cells within the interstitial space. The intracellular compartment is separated from the interstitial space by a cell membrane. This membrane is freely permeable to water but not small or large molecular weight particles. Any particle movement between the interstitium and the cell must occur through some transport mechanism (eg. channel, ion pump, carrier mechanism)
Fluids are in a constant state of flux across the capillary endothelial membrane, through the interstitium and into and out of the cell. The amount of fluid that moves across the capillary "membrane" depends on a number of factors, to include: capillary colloid oncotic pressure, hydrostatic pressure, and permeability.
The natural particles in blood that create COP are proteins—globulins, fibrinogen, and albumin. Albumin is the most numerous and the smallest, approximately 69,000 daltons. The hydrostatic pressure within the capillary is the pressure forcing outward on the capillary "membrane" generated by the blood pressure and cardiac output. Fluid moves into the interstitial space when intravascular hydrostatic pressure is increased over COP, membrane pore size increases, or intravascular COP becomes lower than interstitial COP.
Cellular homeostasis
Transmembrane ion pumps regulate intracellular water maintaining cellular and organelle integrity. The primary active pump for solute transport across the cell membrane is the sodium-potassium pump. Membrane ion pumps also regulate intracellular calcium, hydrogen, chloride, magnesium, glucose and amino acid concentration. These membrane transport systems occur in every cell in every organ, and require energy to function.
Energy required to drive transmembrane ion exchange is supplied by cleavage of adenosine triphosphate (ATP). In contrast to 38 ATP molecules that are produced during aerobic metabolism, anaerobic metabolism produces lactate and only 2 ATP molecules per glucose molecule. Oxygen and glucose are transported from the intravascular space to the cell through a fluid medium. Carried by hemoglobin to the capillaries, oxygen normally diffuses with great ease through the capillary membrane to the cells, most of which are located less than 50 micrometers from a capillary.
The conduit for fluid transport is the vascular system. The heart serves as the pump of the conduit. The primary function of the cardiovascular system is oxygen and glucose delivery to the tissues. Oxygen delivery is dependant on the product of arterial flow and arterial oxygen content. Hemoglobin concentration and its dissociation curve are the prime components of arterial oxygen content.
Arterial flow is a product of cardiac ouput and systemic vascular resistance. Cardiac output is a product of myocardial contraction and heart rate. Sinus node stretch directly increases heart rate in an effort to promote volume ejection. Venous return (preload), as defined by the Frank-Starling law of the heart, increases the stretch of the heart chambers resulting in increased force of contraction. Factors influencing venous return to the heart include: mean circulatory filling pressures, right atrial pressures, and resistance of the arteries.
Blood flow is also influenced by pressure differences and compliance within the vascular circuit as well as viscosity of the fluid medium. Extrinsic and intrinsic regulation of the cardiovascular system also affect blood flow to the tissues. Intrinsic metabolic autoregulation affects local organ blood flow, and is influenced by oxygen availability and removal of metabolic byproducts.
Continuously produced and utilized, ATP production becomes heavily dependant on oxygen availability during high energy output states. Optimum ATP production, therefore, depends on both oxygen delivery (DO2) to the cell and oxygen utilization (VO2) by the cell. Other than oxygen supplementation, reestablishing and maintaining intravascular fluid volume supports maximum oxygen delivery.
Hypovolemic Shock
The consequences of hypovolemic shock have been a well recognized phenomenon for the past 200 years. A significant physiological response occurs when blood volume decreases by 25%, or 10-15ml/kg. Compensatory neuroendocrine responses are initiated for restoring blood volume and meeting metabolic demands occurring during acutely decreased cardiac output states, increasing ATP demands. When perfusion becomes compromised in spite of these mechanisms, decompensatory shock ensues.
Compensatory stage of hypovolemic shock
Acute decreases in intravascular volume causes a decrease in preload (venous return). The baroreceptors initiate an acute compensatory response due to decreased peripheral vagal stimulation and increased sympathetic stimulation. This results in 1) vasoconstriction of the precapillary arteriolar sphinctors, 2) increased heart rate, increased cardiac contractility. Changes in transcapillary pressure gradient (increased intravascular COP, decreased intravascular hydrostatic pressure) will immediately move water from the interstitial space into the intravascular space, and the renin-angiotensin-aldosterone system will lead to increased fluid retention by the kidneys. These mechanisms serve to increase intravascular volume, venous return and therefore cardiac output and arterial flow. Energy is required to sustain this compensatory mechanism, and a supranormal oxygen supply is required for energy production. Substrates required for cellular energy production are provided through the actions of the stress hormones (glucagon, growth hormone, cortisol and ACTH).
These natural neuroendocrine responses can be adequate to compensate for mild to moderate acute decreases in intravascular volume, and produce the compensatory stage of hypovolemic shock. Clinical signs of compensatory shock include tachycardia, bounding pulses, rapid capillary refill time, bright pink to red mucous membranes, and often a normal or elevated blood pressure. The cat does not typically display a compensatory shock response unless there is significant pain present. Rapid intravascular volume expansion is necessary to remove the stimulus inducing this hypermetabolic state.
Should the natural neuroendocrine mechanisms be inadequate to restore baroreceptor stretch, should cardiac dysfunction exist, or should intravsascular volume and systemic vascular resistance be inadequate, cardiovascular decompensation occurs.
Early decompensatory (middle) stage of hypovolemic shock
Continued low cardiac output amplifies sympathetic stimulation, clinically manifesting as significant peripheral vasoconstriction and tachycardia. Arterial blood flow is shunted to preferred organs (i.e. heart and brain) to ensure basic life-support. Cellular oxygen and energy demands increase as vasoconstriction intensifies, and flow is decreased to the skin, mucous membranes and abdominal organs. Oxygen consumption becomes dependant on oxygen delivery, and anaerobic glycolosis results in lactic acid production. Other vasoactive substances produced due to local tissue hypoxia cause a local vasodilation and maldistribution of blood flow.
When chemical mediators produced locally in hypoxic tissues enter the systemic circulation they incite a systemic inflammatory response syndrome, whereby significant vasodilation and damage at the endothelial lining results in increased capillary permeability, further depleting intravascular volume and increasing interstitial fluid. Variable redistribution of blood flow occurs, leading to further consequences, such as compromise of the abdominal organs.
Micro- and macro-ulceration can occur in the gastrointestinal mucosa due to arteriolar vasoconstriction. Gut ischemia and systemic release of inflammatory mediators contribute to the continued pathophysiology resulting from hypovolemic shock. Gastrointestinal flora can translocate into the systemic circulation across the mucosal barrier. The liver may be overwhelmed and unable to filter the portal circulation. Cholestasis and cholecystitis occur. Hypoxic, hepatic venoconstriction and inflammatory mediators stimulating hepatic outflow resistance and portal system expansion, decreasing venous return
Within the kidney, blood will be shunted from the cortical to the juxtamedullary nephrons. Afferent arteriolar constriction limits renal blood flow, resulting in oliguria when the mean arterial blood pressure falls below 60 mmHg. Ischemic renal tubular cells die and form tubular casts obstructing the tubular lumen of the nephron. In the pulmonary circulation, vasoconstriction sustains microcirculatory shunts, impairing oxygen uptake and carbon dioxide diffusion. This multilevel cellular dysfunction places the animal in the early decompensatory (middle) stage of hypovolemic shock. Clinical signs in the dog and cat include: tachycardia (dogs), weak peripheral pulses, pale mucous membrane color, prolonged capillary refill time, and decreased blood pressure. Aggressive volume resuscitation is essential for reducing organ pathology and mortality.
Late decompensatory (final) stage of hypovolemic shock
When severe intravascular volume loss is massive, when earlier compensatory responses are ineffective or inadequately treated, when the insult is severe and overwhelming, or when central pathology blunts the typical compensatory response, late decompensatory shock ensues. Prolonged and severe tissue hypoxia results in the local (intrinsic) responses overriding the sympathetic-mediated vasoconstriction, called autoregulatory escape. This manifests in circulatory collapse and insufficient arterial flow to the brain and heart. The sympathetic center in the brain malfunctions, and the heart cannot sustain either a chronotropic or inotropic response. This is the final common pathway of all forms of shock.
Seperation of capillary endothelial junctions and active transcellular-vesicular transport of proteins result in leakage of albumin and fluids into the interstitium Intracellular and interstitial water content increases as a result of electrogenic pump depression and increased capillary permeability. The resultant hypovolemia and edema affects transport and diffusion of oxygen to the cell, and inadequate ATP production This results in cell membrane dysfunction, cellular swelling and cell death. When a critical number of cells within an organ are dysfunctional or die, failure of that organ occurs.
Clinical signs of this terminal stage are a result of organ failure and include: normal or slow heart rate, no peripheral pulses, white mucous membranes, depressed mentation, absent capillary refill time, and severely low blood pressure. Anuria is evident. Cardiopulmonary arrest is imminent without extreme supportive measures of compromised organs and aggressive cardiovascular resuscitation. After sufficient time and severity, resuscitation efforts may be futile. The key to survival is aggressive resuscitation early in the shock process.
Pain control
It is vital to the maintenance of cardiovascular function and the mental well being of the dog and cat to provide pain control. In the critically ill animal, it is best to titrate analgesics and sedatives to effect, as responses are variable and can be affected by underlying renal and hepatic dysfunction. For mild to moderate pain control, butorphanol 0.2-0.8mg/kg IV q 2-6 hrs) is given initially. For control of severe pain, the combination of injectable opioids oxymorphone (DuPont) 0.05-0.1mg/kg IV or morphine (Steris Labs) 0.1mg/kg IM with diazepam (Steris Labs) 0.2mg/kg IV is effective and reversible.
The fluid resuscitation plan
The fluid resuscitation plan should include the following steps: 1) determine where the fluid deficit lies 2) selection of fluid(s) specific for the patient, 3) determine resuscitation end-points, and 4) determine the resuscitation technique to be employed.
Step 1: Determine where the fluid deficit lies (perfusion vs hydration)
Loss of fluid volume from the intravascular fluid compartment will be manifested by poor perfusion (shock). This is reflected clinically by changes in the heart rate, pulse intensity, capillary refill time, mucous membrane color, and rectal temperature. These are called physical perfusion parameters and, combined with blood pressure, are used clinically to detect intravascular volume deficits. Most animals with an intravascular deficit (poor perfusion) also have concurrent extravascular deficits.
Fluid deficit in the interstitial and intracellular spaces causes clinical signs of dehydration. Physical findings are used to estimate the percentage of dehydration. Semidry oral mucous membranes, normal skin turgor, and eyes maintaining normal moisture indicate 4-5% dehydration. Dry oral mucous membranes, mild loss of skin turgor, and eyes still moist indicate 6-7% dehydration.
As dehydration becomes more severe, significant quantities of fluid shift from the intravascular space into the interstitium causing concurrent perfusion deficits with dehydration. Dry mucous membranes, considerable loss of skin turgor, eyes retracted, and weak rapid pulses (concurrent intravascular deficit) indicate 8-10% dehydration. Very dry oral mucous membranes, complete loss of skin turgor, severe retraction of the eyes, dull eyes, possible alteration of consciousness, and thready weak pulses indicate 12% dehydration.
Step 2. Selection of fluids (crystalloids, colloids or both)
Fluids must be administered that will concentrate within the body fluid compartment where the volume deficit lies. Crystalloids are water based solutions with small molecular weight particles, freely permeable to the capillary "membrane". Colloids are water based solutions that are of a larger molecular weight, too large to freely pass across the capillary "membrane". Colloids are thought of as intravascular volume replacement fluids and crystalloids as interstitial volume replacement solutions.
Crystalloids
The small molecular weight particles in crystalloids are primarily electrolytes and buffers (see Table 1). The sodium concentration of the crystalloid will determine the fluid dynamics of that specific crystalloid. The sodium concentration of the crystalloids compared to the intracellular sodium concentration determines the tonicity of the fluid. When the sodium concentration of the solution is equivalent to that of the cell, the solution is called isotonic. Intravascular administration of isotonic crystalloids (ie. Lactated Ringers, Normosol-R®, Plasmalyte-A®, 0.9% saline) will result in interstitial volume replacement and minimal intracellular fluid accumulation. Over 75% of the isotonic crystalloid administered IV will be in the extravascular space within 1 hr in a normal animal. This is due to the normal fluid shifts between fluid compartments. Hypotonic fluids (eg. 5% Dextrose in water, 1/2 strength saline) will result in intracellular water accumulation and are not used as resuscitation fluids.
Table 1. Crystalloids
Crystalloids are considered balanced when they contain molecules that act as buffers. Acetate, gluconate and lactate are common buffers that are converted to bicarbonate and raise the pH of the solution to normal blood pH (7.4). Most clinical problems will benefit from the use of balanced, isotonic crystalloids (ie lactated Ringers, Normosol-R, Plaasmalyte-A®) as part of the resuscitation fluid plan. Normal saline (0.9%) is isotonic but not balanced, and is used initially for specific clinical problems, to include: hyponatremia, hypernatremia, hypercalcemia, hypochloremic metabolic alkalosis, head trauma, and oliguric renal failure.
Colloids
When colloids are to be administered, it must be decided whether natural colloids (i.e. blood products), a modified biological colloid, a synthetic colloid or a combination is to be used. When the animal requires red blood cells, clotting factors, antithrombin and/or albumin, blood products are necessary. Blood products should be heated in a warm water bath to patient temperature and administered via 18 micron micropore filter. When blood components are not required, initiating fluid resuscitation with modified biological and/or synthetic colloids, can preserve or increase plasma COP.
Hemoglobin-based oxygen-carrying solution (Oxyglobin®) contains stroma free polymerized hemoglobin and is considered a modified biological colloid. In addition to acting as a temporary oxygen-carrying substitute for red blood cells, it provides additional oncotic support and has pressor activity. Its limitations include a short intravascular half-life (40 hours) and interferance with enzyme chemistry analyses. Red cell replacement may still be required if significant anemia is present. Other side effects that may occur include pulmonary edema, vomiting and diarrhea.
Synthetic colloids were developed to provide timely and convenient fluid resuscitation while avoiding the problems encountered with rapid natural colloid infusions. They provide an increase in COP beyond what is attainable with natural colloids and can be used in conjunction with whole blood or plasma. They are not, however, to be considered a substitute for blood products when albumin, hemoglobin, antithrombin, or coagulation proteins are needed.
The benefits of synthetic colloids are related to their ability to attract and retain water in the intravascular space. Larger molecular weight colloids (i.e. hydroxyethyl starches and dextran 70) will retain water longer, maintaining oncotic pressure as they are broken down into smaller particles prior to elimination. This characteristic is advantageous when sustained volume support is required during increased capillary permeability, and when there is less tolerance of rapid intravascular volume increases (e.g. during brain and pulmonary injury, cardiac insufficiency, or in hypovolemic cats).
Fluid selection
Interstitial and intracellular volume deficits (dehydration) are replaced by the administration of crystalloids. Intravascular volume (perfusion) deficits can also be replaced with crystalloids alone. However, when large quantities of isotonic crystalloids are rapidly administered intravenously, there is an immediate increase in intravascular hydrostatic pressure, a decrease in intravascular COP and extravasation of large fluid quantities into the interstitial spaces.
By administering colloids in conjunction with crystalloids during fluid resuscitation of perfusion deficits, less total fluid volume is required, there is less tendency toward fluid overload, and resuscitation times are shorter. Colloid administration is combined with crystalloid administration, allowing the crystalloid volume to be decreased by 40-60%. The information provided in Table 2 will guide colloid selection for the individual patient. When there is increased capillary permeability and loss of albumin through the capillary membrane, hetastarch or stroma free hemoglobin are the colloids of choice for intravascular volume resuscitation. Persisting in giving crystalloids alone will result in significant fluid flux out of the capillary and into the interstitial fluid compartment.

Table 2. Colloids
Step 3: Determine resuscitation end-points (supranormal vs. hypotensive)
There are no effective "standard" formulas for crystalloid or colloid infusion that will guarantee complete volume resuscitation in a small animal patient. Variables such as renal function, presence of a third body fluid space, brain injury, lung injury, heart disease or failure, and/or closed cavity hemorrhage will require that fluid resuscitation rate and volumes be individualized for the patient. Fluid quantities should be administered sufficient to reach desire end-points of resuscitation. The end-points typically reflect the perfusion status of the animal, and include heart rate, blood pressure, central venous pressure, mucous membrane color, capillary refill time, and pulse intensity.
Shock will cause a depletion of cellular energy stores with subsequent cellular and organ dysfunction. Restoring the circulation to "normal", with normal oxygenation and normal perfusion parameters, may not be enough to allow sufficient ATP production for repair as well as maintenance. When a patient is suspected of having a SIRS related disease process (vasodilation, increased capillary permeability, depressed cardiac output) resuscitation end-points are chosen for supranormal resuscitation (see Table 3). The goal is to deliver oxygen and glucose to the cells in concentrations higher than normal to promote sufficient energy production for both repair and maintenance of the cells.

Table 3. End-point resuscitation
There are clinical situations, however, when supranormal resuscitation can be detrimental to the animal. Increased vessel wall tension can dislodge a life-saving clot in the vasculature of a traumatized animal, resulting in significant hemorrhage. Brain and lung edema or hemorrhage can be worsened by aggressive and sudden increases in hydrostatic pressure. Hypotensive resuscitation provides end-points that are at the lower limit of normal (see Table 3). The goal is to administer the smallest volume of fluids possible to successfully resuscitate the intravascular compartment while minimizing extravasation of fluids into the interstitium (esp. brain or lungs), titrating the amount of preload stretching a potentially disabled heart, and reducing the probability of disturbing clot formation. Small volume resuscitation techniques should be used to reach hypotensive resuscitation end-points.
Step 4: Determine the resuscitation technique to be employed (large volume vs small volume)
An algorithm for selecting and employing specific resuscitation techniques for the dog are shown in Figure 1 and for the cat in Figure 2. Dogs in hypovolemic shock that require supranormal end-point values can benefit from large volume resuscitation techniques. Typically, an initial infusion of 20-50 ml/kg of balanced isotonic crystalloids is given followed by a 5-15 ml/kg dose of hetastarch or dextran –70. When stroma free hemoglobin is selected as the colloid, the dose is 5 ml/kg. Additional colloids can be administered using small volume intravascular resuscitation techniques if perfusion has not improved to the desired supranormal end-points after the initial large volume dose of fluids.
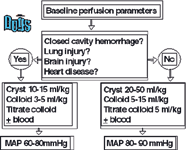
Whole blood products can be administered by large volume resuscitation techniques in catastrophic hemorrhagic situations. However, initial administration of stroma free hemoglobin will provide time for a slower administration of whole blood and less chance for transfusion reaction from rapid whole blood administration.
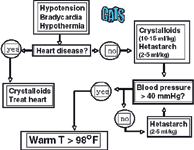
Small volume resuscitation techniques are recommended in the hypovolemic cat and any dog with closed cavity hemorrhage, head injury, pulmonary contusions or edema, cardiogenic shock, or oliguric renal failure. An initial dose of balanced isotonic crystalloids (10-15 ml/kg for dogs; 5-10 ml/kg for cats) is given. Either hetastarch or dextran-70 is then administered (5 ml/kg in dogs; 2-5 ml/kg in cats) over 1-5 minutes. The perfusion parameters are reassessed and the initial ml/kg bolus repeated as needed until the end-point of resuscitation is reached. When stroma free hemoglobin is used as the colloid, a dose of 2-5 ml/kg is given for dogs. Stroma free hemoglobin has not been approved for use in cats but has been used successfully by the author when titrating with doses of 1-5 ml/cat (0.25-1.0 ml/kg) slowly over 5 minutes.
Hypothermia, especially in the cat, can significantly limit the cardiovascular response to fluid resuscitation. Active external warming with water circulating blankets should occur once fluid resuscitation has been initiated (see Figure 2). It has been the author's experience that aggressive volume administration without active warming of the hypothermic cat can result in pulmonary edema despite continued hypotension.
Assessment of resuscitation efforts
Once the fluid therapy plan has been employed, it is critical that there is on-going assessment of the patient. When an adequate amount of fluids has been administered and there is failure to reach reasonable resuscitation end-points, several etiologies are considered; inadequate volume administration; on-going hemorrhage; third body fluid spacing, heart disease; severe vasodilation; vasoconstriction; hypoglycemia; hypokalemia, arrhythmias, and brain pathology. These variables should be rapidly assessed and corrected. If a central venous pressure (CVP) is available, it should be checked to see if it is near the end-points assigned in table 3. If not, or if no CVP is available, a fluid challenge can be given. This typically consists of a 10-15 ml/kg bolus of crystalloids and 5 ml/kg bolus of hetastarch. If the perfusion parameters improve with this challenge, then the likely cause of the non-responsive shock is inadequate volume.
If the fluid volume appears adequate and the patient is still hypotensive, vasopressors can be used. Oxyglobin® can be given at above dosages if it has not been used in the fluid plan so far. If the stroma free hemoglobin has failed to increase the blood pressure, then dopamine at 5-15 ug/kg/min is administered as a constant rate of infusion. This is weaned once the animal can maintain the blood pressure for a minimumof 4 hours.
Volume maintenance
Maintaining intravascular fluid after resuscitation from hypovolemic shock and during systemic inflammatory response syndrome (SIRS) disease conditions causing increased capillary permeability can be a challenge. Hetastarch or Oxyglobin® can be administered as a constant rate infusion of 0.5-1 ml/kg/hr in the dog, or 0.25-1.0 ml/kg/hr in the cat. The dose is adjusted to maintain an adequate mean arterial pressure and central venous pressure. The amount of crystalloid administered with colloids must be reduced by 40-60% of what would be administered if crystalloids were used alone.
References available upon request