Skills Laboratory: How to make a high-quality slide from a fine-needle aspirate
Follow this step-by-step guide to prepare a high-quality slide, increasing the value of its interpretation.
Fine-needle aspiration and cytologic examination remain a basic yet valuable skill in the expanding diagnostic veterinary world. When a new lump appears on a patient, performing fine-needle aspiration and cytologic examination are both appropriate and informative but have clear limitations that must be discussed with owners so their expectations are realistic.
I explain to my clients that cytology is the review of cells obtained from a very small needle, whereas a biopsy involves harvesting a portion of tissue that yields many more cells to be examined and recognized, thus providing more information. Explaining this difference is important since many clients will assume these techniques are interchangeable and may be disappointed if the cytologic findings are not definitively diagnostic.
ADVANTAGES OF CYTOLOGY
I encourage practitioners to start with fine-needle aspiration and cytologic examination for several reasons.
- Fine-needle aspiration is minimally invasive and does not require general anesthesia or even sedation, so the risk to the patient for severe bleeding or infection, for example, is minimal.
- Although a diagnosis is not guaranteed, many diseases or conditions-such as lipomas, sebaceous cysts, inflammation, and most round cell tumors (e.g. mast cell tumors)-can be initially diagnosed with cytologic examination results.
- Performing fine-needle aspiration and cytologic examination is technically easier and less expensive than performing a biopsy and histologic examination.
- Cytologic findings may help with staging a cancer patient and planning the treatment protocol; the findings are especially beneficial with the mapping and monitoring of tumors over consecutive examinations.
LIMITATIONS OF CYTOLOGY
One limitation of cytologic examination is the lack of a complete set of special stains that may be necessary to identify the tissue of origin. Many more special stain options are available with biopsied tissue. In addition, cytologic examination results may not always provide a diagnosis. In some cases, a tumor type may be suspected, which may be helpful, but margin evaluation and grade as well as important histopathologic factors such as lymphatic and vascular invasion may not be determined. Mammary tumors and splenic tumors rarely benefit from fine-needle aspiration and cytologic examination, and experience has taught me that many of the findings are nondiagnostic or the procedure hastens intra-abdominal bleeding-or both.
FINE-NEEDLE ASPIRATION TECHNIQUES
There are two fine-needle aspiration methods: the needle-on method or the needle-off method. I prefer the needle-off procedure for obtaining samples except when obtaining ultrasound-guided aspirations, in which case the needle-on technique helps with guidance. For some masses, exfoliation is poor, and gentle aspiration with the needle on is helpful to obtain a cellular sample.
I prefer 22-ga, 1-in needles and 6-ml syringes for most cutaneous preparations. For ultrasound-guided fine-needle aspirations, I prefer 27-ga, 1 1/2-in needles and 6-ml syringes. The small aperture of the 27-ga needle allows for a diagnostic yield of representative cells while also minimizing discomfort, thus allowing most of my ultrasound-guided fine-needle aspirations to be obtained with minimal restraint and often without sedation.
Step 1

Prepare for the procedure by laying out your slides and gathering your needle and syringe. I generally make five or six slides per site but have more slides ready in case they are needed.
Step 2
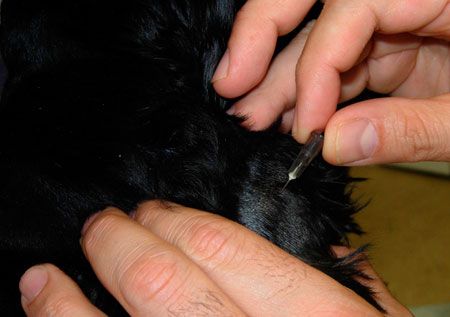
Clean the site with saline solution or alcohol, and then guide the needle into the mass. The center may not be the best location because of possible necrosis of the tissue or cystic pockets of fluid, so aim for multiple angles of insertion. The goal is to fill the needle with tissue and then to push that material with the syringe onto the slides. I do not recommend using the “sewing machine,” or “woodpecker,” technique because it may lead to excessive trauma and post-procedure hemorrhage as well as to contamination of the sample with red blood cells.
Step 3
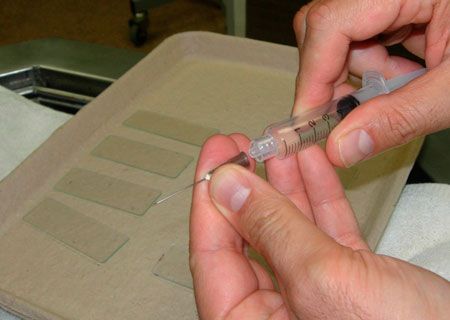
Reattach the syringe after drawing air in.
Step 4

Place the needle tip to one side of the slide, and push the syringe in one swift motion to expel the sample.
Step 5
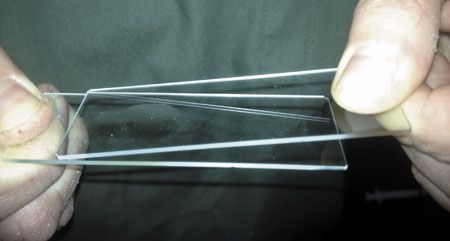
Place one edge of a new slide on the deposited sample, and with a smooth, gentle motion guide the new slide across the sample, creating an even distribution of cells on the slide. Thick tissue needs to be thinned out, so multiple slides should always be available. Too thick a sample on a slide will obscure an accurate cytologic examination because of excessive layering of cells. Sometimes cytology provides enough tissue for histopathology, so place any excessively thick tissue into a cassette and formalin fixation.
< Back
SUBMITTING SLIDES
Prepare multiple slides, and send more than you think you will need to the clinical pathologist. Sometimes only one slide out of a batch of slides will be diagnostic. When submitting multiple slides, consider staining one slide in the hospital to confirm a representative cluster of intact cells. I will often submit the stained slide to the pathologist to reveal what I am seeing. In addition, make sure to provide an accurate, clear patient history and the site of origin to the pathologist.
CONCLUSION
Fine-needle aspiration and cytologic examination can be a rewarding test, but making diagnostic-quality slides takes practice. Poorly prepared slides will limit any pathologist's ability to interpret the findings and may jeopardize the pathologist's ability to provide a diagnosis, thus limiting your ability to formulate a treatment plan or coordinate a referral for the patient.