Three emerging vector-borne diseases in dogs and cats in the United States
Several vector-borne diseases in dogs and cats appear to be emerging in the United States, including babesiosis, cytauxzoonosis, bartonellosis, leishmaniasis, hepatozoonosis, and feline ehrlichiosis. This article focuses on babesiosis, cytauxzoonosis, and bartonellosis, which have been reported with increased frequency in the United States over the past decade.
Several vector-borne diseases in dogs and cats appear to be emerging in the United States, including babesiosis, cytauxzoonosis, bartonellosis, leishmaniasis, hepatozoonosis, and feline ehrlichiosis. This article focuses on babesiosis, cytauxzoonosis, and bartonellosis, which have been reported with increased frequency in the United States over the past decade.1-20
It is not always clear whether the prevalence of these infections is truly increased or whether the more frequent diagnoses are due to increased awareness and improved diagnostic testing. In some cases of emerging diseases, such as with bartonellosis, the reason the disease is being diagnosed more often may simply be related to the discovery of its existence in dogs and our increased awareness. In other cases, such as with babesiosis and cytauxzoonosis, the reasons behind the emergence are not fully elucidated, but international travels, possible genetic mutations of the host or pathogen, and increased clinician awareness are suspected causes. Regardless of the reasons for their emergence, practitioners need to consider these infections in their patients. This article reviews the clinical signs and current recommendations for diagnosing and treating these three infections.
CANINE BABESIOSIS
Bovine babesiosis was the first infectious disease for which tick transmission was documented. After this discovery in 1893, bovine babesiosis was eventually eradicated in the United States. Canine babesiosis was first described in South Africa in the late 1800s and was first reported in the United States in 1934.21 An increased number of cases of canine babesiosis have been reported in the United States since the late 1990s.1-4,22
It appears that breed predispositions for canine babesiosis exist in the United States. Most Babesia canis infections reported in the United States have occurred in greyhounds, and most Babesia gibsoni cases have occurred in American pit bull terriers.1,4,23,24 The underlying causes for these predispositions are unknown, but lifestyle and housing conditions that lead to an increased risk of exposure are thought to be more likely than a genetic predisposition for infections. Besides ticks, perinatal transmission, direct dog-to-dog transmission by bite wounds, and mechanical transmission are the predominant modes thought to be involved.25
Classification
Babesia species are intraerythrocytic protozoan parasites that can be transmitted by ticks. Babesia species have been classified historically based on their size and the mammalian species they infect. Large Babesia species are 3 to 6 µm in length, and small Babesia species measure 1 to 3 µm. In dogs, B. canis is the most commonly identified large Babesia species. Babesia canis includes three subspecies: B. canis vogeli, B. canis canis, and B. canis rossi. These three subspecies are genetically distinct, are transmitted by different vectors, and have different geographic distributions and varying degrees of pathogenicity.26-28 Babesia canis vogeli has a worldwide distribution, is transmitted by Rhipicephalus sanguineus, and is considered to be mildly to moderately pathogenic.23,24 Babesia canis canis is found primarily in Europe, is transmitted by Dermacentor reticulatus, and is moderately pathogenic.29-32 Babesia canis rossi is endemic to Africa, is transmitted by Haemaphysalis leachi, and is a virulent subspecies.27,33,34 At least one other species of large Babesia has been identified in a dog with babesiosis.35
At least three genetically distinct small Babesia species can infect dogs.36-38 Babesia gibsoni has a worldwide distribution, is transmitted by Haemaphysalis species ticks, and has variable degrees of virulence ranging from subclinical infections to severe life-threatening disease.39,40 A second small Babesia species was identified in southern California in 1991 and was also referred to as B. gibsoni.41 However, genetic data suggest that this organism is more closely related to some Theileria species than it is to Babesia species.36,37 The official phylogenic description of this organism is yet to be reported and is referred to as the western piroplasm in this article. The western piroplasm has only been reported in Southern California, the vector is unknown, and it is a virulent pathogen. The third small piroplasm is tentatively named Theileria annae.38 Theileria annae is endemic to Spain. The vector is suspected to be Ixodes hexagonus, and it appears to be a virulent pathogen.42-44
Clinical disease
Anemia and thrombocytopenia are the most consistent clinicopathologic abnormalities detected in dogs with babesiosis independent of which Babesia species causes the infection.45 Both the anemia and thrombocytopenia are associated with immune-mediated destruction and can be nearly indistinguishable from idiopathic immune-mediated cytopenias. However, variations in the presentations among Babesia species infections do occur. Babesia canis vogeli infection is most commonly associated with anemia and thrombocytopenia, and severe clinical disease is more frequently detected in puppies than in adults.23,24 Adults can have subclinical infections. Babesia canis canis is associated with disease in both puppies and adult dogs, and signs associated with anemia are the predominant presenting complaint.29,31,32 However, many adult carriers of B. canis canis are relatively asymptomatic.46 Babesia canis rossi is also associated with clinical disease in both puppies and young adults.33,47 Besides the classic signs associated with hemolytic anemia, B. canis rossi is the subspecies of B. canis that seems to be associated with the most diverse range of clinical diseases and presentations. Some dogs have neurologic signs that can either be associated with hypoglycemia or possibly cerebral ischemia. Another subset of dogs have severe metabolic acidosis and azotemia.34,48 Atypical babesiosis associated with B. canisrossi is usually associated with a poor prognosis.
As with the Babesia canis subspecies, anemia and thrombocytopenia are the most consistent hematologic abnormalities detected in dogs infected with small piroplasms. Babesia gibsoni infection can have a diverse range of clinical manifestations and does not appear to be associated with age.49 Some dogs with B. gibsoni infection suffer from fatal anemia while other dogs that have presumably recovered from the acute stages of infection have normal hematocrit results. In some studies, thrombocytopenia was a more common hematologic abnormality than anemia was.4 Infection with the western piroplasm detected in Southern California was associated with a mortality rate of nearly 60%.41,50 Theileria annae infection is associated with azotemia in up to 40% of the cases reported, and azotemia was associated with mortality in those cases.42,43
Diagnosis
No one test is clearly the best for definitively diagnosing babesiosis as none of the tests are 100% sensitive or 100% specific. Three basic types of tests are available to help diagnose babesiosis: light microscopy, serology, and molecular testing.51 Light microscopy (Figures 1A & 1B) is widely available and has a high specificity when the blood smears are evaluated by properly trained individuals, but it probably has the lowest sensitivity of the available tests.52 Because of the high degree of morphologic variation of the piroplasms during natural infections, microscopy cannot always allow accurate identification of the Babesia species or subspecies present.26,36
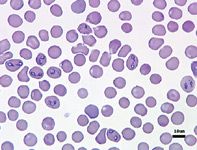
Figure 1A. Canine red blood cells infected with Babesia canis (Wright's-Giemsa; 500X).
Serologic testing detects antibodies that react with Babesia species antigens and includes immunofluorescence assays (IFAs) and enzyme-linked immunosorbent assays (ELISAs). Although many ELISA-based tests for canine babesiosis have been described, an IFA against whole parasite preparations is the primary serologic test available to practitioners. Since an IFA relies on whole parasite preparations and each laboratory uses different strains for antigen preparation, inherent variability can exist among laboratories. Serologic testing appears to be sensitive, but the sensitivity is not 100% as evidenced by the recognition of babesiosis in dogs that do not have detectable antibodies against Babesia species antigens.22,23,35 Individual laboratories establish cutoff titers indicative of exposure to Babesia species, so do not compare the results of different laboratories.53
Testing for Babesia species DNA in clinical samples has improved our ability to confirm Babesia species infections and is the only way to definitively determine the species or strain of Babesia present.36 Most of these tests are based on the polymerase chain reaction (PCR), which exponentially amplifies a specific fragment of DNA.54-56 A positive PCR test result is indicative of current infection, when the appropriate controls have been used. As with IFAs, individual laboratories perform different PCR tests that have different sensitivities and specificities. It is imperative that the laboratory performing the PCR is strictly adhering to the practices used to avoid contamination and is using appropriate positive and negative controls. Unfortunately, not many studies have been done to determine the clinical sensitivity of PCR testing for diagnosing canine babesiosis. One PCR test had an overall sensitivity of 87% when used to detect B.gibsoni in asymptomatic carrier dogs and detected 100% of the carriers when two consecutive tests were performed 30 days apart.5

Figure 1B. Canine red blood cells infected with Babesia gibsoni (Wright's-Giemsa; 750X).
Treatment and prevention
Imidocarb dipropionate is the only drug approved by the FDA for treating canine babesiosis. The labeled dosage is 6.6 mg/kg given intramuscularly; the dose is repeated in two weeks for a total of two treatments. The most commonly reported side effects are pain at the injection site and cholinergic signs, such as salivation, defecation, and panting. Pretreatment with atropine (0.022 mg/kg subcutaneously 15 to 30 minutes before imidocarb administration) may reduce cholinergic signs. Imidocarb is effective for treating all subspecies of B. canis and is likely to eliminate B. canis infections completely.57-60 Imidocarb reduces morbidity and mortality when used to treat B. gibsoni infections but is not effective in eliminating the infections.5,61 No extensive reports exist on the use of imidocarb for treating infection with the western piroplasm or T. annae. Recent studies have shown that treating dogs with B. gibsoni infection with a combination therapy of atovaquone (Mepron–GlaxoSmithKline; 13.5 mg/kg orally t.i.d. given with a fatty meal for 10 days) and azithromycin (10 mg/kg orally once a day for 10 days) resulted in either elimination of infection or the suppression of parasitemia below the limit of detection.5 In my experience, early diagnosis and treatment with appropriate antiprotozoal drugs result in the highest rate of remission. The anemia and thrombocytopenia associated with babesiosis are immune-mediated, so concurrent treatment with immunosuppressive drugs is often instituted. However, in my experience, prolonged immunosuppression (weeks to months) before specific antiprotozoal therapy has resulted in a worse prognosis for remission.
Some studies have documented the prevention of babesiosis with imidocarb administration before experimental challenge infection.59 This approach is not practical in most cases. Reduced exposure to risk factors for transmission is recommended and is likely to reduce the chances of infection. These precautions include using acaricides, removing ticks daily, screening brood bitches (greyhounds and American pit bull terriers), screening blood donors, sterilizing needles and surgical equipment, and preventing dogfights.
FELINE CYTAUXZOONOSIS
Cytauxzoonosis is a tick-transmitted disease in cats caused by the protozoan parasite Cytauxzoon felis. Feline cytauxzoonosis was first described in two cats from Missouri in 1975, and since that time cytauxzoonosis has been recognized throughout much of the southeastern and midwestern United States.6,62-66 Wild felids such as bobcats (Lynx rufus) are considered to be the main reservoir for C. felis.67,68 Dermacentor variabilis has been demonstrated to transmit C. felis from bobcats to domestic cats.69 Only trans-stadial transmission of C. felis in D. variabilis has been documented.69 In domestic cats, C. felis undergoes a tissue or schizogenous phase followed by an erythrocytic phase. Clinical disease and death are attributed mainly to the tissue phase of the infection.
Clinical disease
Two distinct clinical presentations of cytauxzoonosis occur in domestic cats. Some cats have acute severe illness while others have mild or absent clinical disease.70 Cats with acute severe illness typically are presented with a one- to three-day history of anorexia and lethargy.6 Occasionally owners will notice dyspnea as the primary sign, or the cat may be found in a recumbent state. Physical examination often reveals one or more of the following: fever, icterus, lymphadenopathy, hepatomegaly, or splenomegaly.6,12,71 In end-stage disease, cats often become hypothermic.6 Some cats will vocalize as if in pain or distress.70
Pancytopenia is considered to be the classic hematologic abnormality associated with cytauxzoonosis, but cats may only present with decreases in one or two cell lines.6,70,72 Anemia and thrombocytopenia are the most commonly reported cytopenias. The anemia is often nonregenerative at the time of presentation because of the acute nature of the disease. Common serum chemistry profile abnormalities detected in these cats include hyperbilirubinemia and increased liver enzyme activity. Most clinical signs in acute cytauxzoonosis are attributed to the occlusion of small vessels by schizont-laden macrophages.6,70,72
Less commonly, cats are presented with mild clinical signs or without any signs of clinical illness. Many of these cases have been identified in the same household or area as cats that have developed severe or fatal illness. The reasons for the distinctly different manifestations of infection are unknown. Some researchers speculate that it may be due to a genetic mutation of the C. felis organisms rendering them less pathogenic, while others speculate that the immune response in some cats results in less severe clinical disease.
Diagnosis
Cytology and histopathology remain the only commercially available tests for diagnosing C. felis infection. Serologic and molecular tests have been described but have only been available for research.12,73 The tissue phase of the infection can often be diagnosed by identifying schizont-infected macrophages in cytologic preparations of tissues such as the lymph nodes, liver, or spleen (Figure 2). These infected macrophages will occasionally be seen in the feathered edge of stained thin blood smears. The erythrocytic phase is characterized by the classic signet-ring-shaped piroplasms within red blood cells (Figure 3). The intracellular piroplasms can be differentiated from hematotropic Mycoplasma species, which have an epicellular location. Keep in mind that the erythrocytic phase is not always present or detected at the time of presentation. So in endemic regions, perform a cytologic examination of the lymph nodes, liver, or spleen in cats that are presented with acute febrile illness, especially when it is accompanied by icterus or cytopenias.
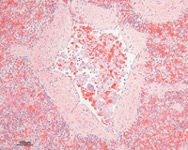
Figure 2. Schizont-laden macrophages occluding a splenic arteriole in a cat with cytauxzoonosis (hematoxylin-eosin; 200X).
Treatment and prevention
No treatments have demonstrated consistent efficacy against C. felis infection or consistent decreases in morbidity and mortality. Most clinicians agree that aggressive supportive care with anticoagulants such as heparin and intravenous fluid therapy is the standard of care for cats with or suspected of having cytauxzoonosis.70 The use of antiprotozoal drugs is somewhat controversial. There are a few reports of using imidocarb dipropionate or diminazene aceturate to treat C. felis infection, but the effect of these treatments on survival is not clear.12,74 Some naturally infected cats that survived infection were treated with imidocarb, but at least as many cats that survived either were not treated with imidocarb or received no treatment at all.12 If you institute treatment with imidocarb, pretreat with atropine to minimize cholinergic side effects. The dose of imidocarb used in cats (2 mg/kg intramuscularly once) is lower than that used in dogs.74

Figure 3. Feline red blood cells infected with Cytauxzoon felis (Wright's-Giemsa; 750X).
Because no consistently effective treatment for C. felis infection is available, disease prevention is of the utmost importance. Tick transmission appears to be the primary route of transmission, so avoiding exposure to ticks and promptly removing ticks are likely to be important to prevent transmission. Ideally, cats should be housed indoors, especially during the spring and summer when ticks are most active.
CANINE BARTONELLOSIS
Bartonella species are fastidious facultative intracellular gram-negative bacteria. Many Bartonella species have been demonstrated to be transmitted by insect vectors.75,76 The first Bartonella species was isolated in 1909 and was determined to be the agent of human disease in South America.77 Bartonella species are responsible for many diseases in both immunocompetent and immunocompromised people, including Oroya fever, trench fever, cat-scratch disease, bacillary angiomatosis, and bacillary peliosis.
Most Bartonella species have been identified in the past 10 years.76 One characteristic of Bartonella species infections is their ability to cause persistent infections in their mammalian hosts that can last for years. Canine Bartonella species infection was first described 1995.14 Since that time, five species of Bartonella have been identified in dogs: Bartonella vinsonii subsp berkhoffi, Bartonella clarridgeiae, Bartonella washoensis, Bartonella henselae, and Bartonella elizabethae.78-82
Clinical disease
The full spectrum of canine disease caused by Bartonella species has yet to be elucidated. Infective endocarditis is the best-characterized disease associated with canine bartonellosis.14,20,81,82 The manifestations of infective endocarditis caused by Bartonella species do not appear to be different from those caused by other bacteria. The most common presenting complaints include lameness and dyspnea, presumably due to immune-mediated polyarthritis and congestive heart failure, respectively. Physical examination findings in dogs with endocarditis caused by bartonellosis typically include heart murmur, fever, and dyspnea, with many dogs presenting in congestive heart failure.20
In the cases reported in the literature, the most common hematologic abnormalities include mild thrombocytopenia, leukocytosis, and anemia. Common serum chemistry profile abnormalities include mild hypoalbuminemia, mild to moderately increased liver enzyme activity, and azotemia.20 Unfortunately, most cases reported have not clearly defined whether or not the azotemia was of renal or prerenal origin. Proteinuria was the most common abnormal finding on urinalysis.
Many other conditions and abnormalities have been associated with bartonellosis in dogs, but a cause-and-effect relationship has not been clearly established.83 Some of these findings include granulomatous lymphadenitis, granulomatous rhinitis, granulomatous hepatitis, and peliosis hepatis.19,80,84,85 A recent study in dogs that had anti-Bartonella species antibodies reported thrombocytopenia and neutrophilic leukocytosis in 50% of the dogs.83 Further studies are needed to fully elucidate the clinical manifestations associated with bartonellosis in dogs.
Diagnosis
Serology, PCR testing, and culture are the three most widely available tests for canine bartonellosis. Serology is only commercially available for B. vinsonii and B. henselae and is performed by using an IFA-based test or a Western blot test. Both tests appear to be sensitive for detecting exposure to Bartonella species, but the presence of antibodies against Bartonella species antigens may not always correlate with current infection. The PCR test detects a specific fragment of Bartonella species DNA and is indicative of current infection, but false negative results may occur when bacteria are present in low numbers. Culture is highly specific, but the sensitivity is poor because of the low numbers of circulating bacteria and their fastidious nature, which makes them difficult to grow. Because both the true prevalence of bartonellosis in the canine population and the full spectrum of disease caused by these organisms are unknown, interpret positive test results with caution in regard to whether bartonellosis is the cause of any underlying disease.
Treatment and prevention
The best treatment for canine bartonellosis has yet to be established, as no formal studies have been performed to determine the efficacy of antibiotic treatments against Bartonella species in dogs. Because of experience with treating bartonellosis in other species, dogs have been treated with a variety of antibiotics, including amoxicillin trihydrate-clavulanate potassium, azithromycin, doxycycline, and enrofloxacin.83 In many of the cases reported, the clinical signs resolved after antibiotic treatment, but whether this is because the bartonellosis resolved is difficult to determine because of concurrent treatments and diseases. Many clinicians are recommending treatment with azithromycin (5 to 10 mg/kg orally once a day for five days, followed by 5 to 10 mg/kg orally every other day for six weeks).
Since the natural route of transmission for Bartonella species in dogs is unknown, the best method of prevention is unknown. In one study, tick exposure and outdoor environments were associated with bartonellosis, as was concurrent seroreactivity against several tick-borne diseases such as ehrlichiosis and babesiosis.15 These factors implicate tick transmission for bartonellosis, so acaricides and daily tick removal are recommended.
Bartonella species are recognized as potential zoonotic pathogens, so proper client education is important when bartonellosis is diagnosed in a dog. A few cases of human bartonellosis have occurred in which the only animal contact was with a dog.86,87 The possible modes of transmission between dogs and people are unknown, but avoiding bites and scratches and providing appropriate flea and tick control to dogs should be recommended.
Adam Birkenheuer, DVM, PhD, DACVIM (internal medicine)
Department of Clinical Sciences
North Carolina State University
College of Veterinary Medicine
Raleigh, NC 27607
REFERENCES
1. Birkenheuer AJ, Levy MG, Savary KC, et al. Babesia gibsoni infections in dogs from North Carolina. J Am Anim Hosp Assoc 1999;35:125-128.
2. Irizarry-Rovira AR, Stephens J, Christian J, et al. Babesia gibsoni infection in a dog from Indiana. Vet Clin Pathol 2001;30:180-188.
3. Kocan AA, Kjemtrup A, Meinkoth J, et al. A genotypically unique Babesia gibsoni-like parasite recovered from a dog in Oklahoma. J Parasitol 2001;87:437-438.
4. Macintire DK, Boudreaux MK, West GD, et al. Babesia gibsoni infection among dogs in the southeastern United States. J Am Vet Med Assoc 2002;220:325-329.
5. Birkenheuer AJ, Levy MG, Breitschwerdt EB. Efficacy of combined atovaquone and azithromycin for therapy of chronic Babesia gibsoni (Asian genotype) infections in dogs. J Vet Intern Med 2004;18:494-498.
6. Hoover JP, Walker DB, Hedges JD. Cytauxzoonosis in cats: eight cases (1985-1992). J Am Vet Med Assoc 1994;205:455-460.
7. Walker DB, Cowell RL. Survival of a domestic cat with naturally acquired cytauxzoonosis. J Am Vet Med Assoc 1995;206:1363-1365.
8. Fayer R. Global change and emerging infectious disease. J Parasitol 2000;86:1174-1181.
9. Lethgo JH. Tick-borne disease kills cats in Northeast county. Tennessean 2000;June 9.
10.C. felis spreads without cure. DVM Newsmagazine 2002;35.
11. Sodders LM. Deadly cat disease appears in Topeka. Topeka Capital-Journal 2000;June 20.
12. Meinkoth J, Kocan AA, Whitworth L, et al. Cats surviving natural infection with Cytauxzoon felis: 18 cases (1997-1998). J Vet Intern Med 2000;14:521-525.
13. Le J, Valensizi A, Birkenheuer AJ, et al. The expanding geographical distribution of Cytauxzoon felis, in Proceedings. NC State Univ Res Forum 2004.
14. Breitschwerdt EB, Kordick DL, Malarkey DE, et al. Endocarditis in a dog due to infection with a novel Bartonella subspecies. J Clin Microbiol 1995;33:154-160.
15. Pappalardo BL, Correa MT, York CC, et al. Epidemiologic evaluation of the risk factors associated with exposure and seroreactivity to Bartonella vinsonii in dogs. Am J Vet Res 1997;58:467-471.
16. Kordick DL, Breitschwerdt EB. Persistent infection of pets within a household with three Bartonella species. Emerg Infect Dis 1998;4:325-328.
17. Breitschwerdt EB, Atkins CE, Brown TT, et al. Bartonella vinsonii subsp. berkhoffi and related members of the alpha subdivision of the Proteobacteria in dogs with cardiac arrhythmias, endocarditis, or myocarditis. J Clin Microbiol 1999;37:3618-3626.
18. Barnes A, Bell SC, Isherwood DR, et al. Evidence of Bartonella henselae infection in cats and dogs in the United Kingdom. Vet Rec 2000;147:673-677.
19. Pappalardo BL, Brown T, Gookin JL, et al. Granulomatous disease associated with Bartonella infection in 2 dogs. J Vet Intern Med 2000;14:37-42.
20. MacDonald KA, Chomel BB, Kittleson MD, et al. A prospective study of canine infective endocarditis in northern California (1999-2001): emergence of Bartonella as a prevalent etiologic agent. J Vet Intern Med 2004;18:56-64.
21. Eaton P. Piroplasma canis in Florida. J Parasitol 1934;20:312-313.
22. Birkenheuer AJ, Levy MG, Stebbins M, et al. Serosurvey of antiBabesia antibodies in stray dogs and American Pit Bull Terriers and American Staffordshire Terriers from North Carolina. J Am Anim Hosp Assoc 2003;39:551-557.
23. Breitschwerdt EB, Malone JB, MacWilliams P, et al. Babesiosis in the Greyhound. J Am Vet Med Assoc 1983;182:978-982.
24. Taboada J, Harvey JW, Levy MG, et al. Seroprevalence of babesiosis in Greyhounds in Florida. J Am Vet Med Assoc 1992;200:47-50.
25. Boozer AL, Macintire DK. Babesia gibsoni. Compend Cont Ed Pract Vet 2005;27:33-42.
26. Uilenberg G, Franssen FF, Perie NM, et al. Three groups of Babesia canis distinguished and a proposal for nomenclature. Vet Q 1989;11:33-40.
27. Hauschild S, Schein E. The subspecies specificity of Babesia canis [German]. Berl Munch Tierarztl Wochenschr 1996;109:216-219.
28. Zahler M, Schein E, Rinder H, et al. Characteristic genotypes discriminate between Babesia canis isolates of differing vector specificity and pathogenicity to dogs. Parasitol Res 1998;84:544-548.
29. Martinod S, Laurent N, Moreau Y. Resistance and immunity of dogs against Babesia canis in an endemic area. Vet Parasitol 1986;19:245-254.
30. Moreau Y, Martinod S, Fayet G, et al. Epidemiologic and immunoprophylactic aspects of canine babesiosis in France. In: Babesiosis of domestic animals and man. Boca Raton, Fla: CRC Press Inc., 1988;191-196.
31. Pages JP, Vidor E, Trouillet JL, et al. Clinical, haematological and serological description of 133 cases of babesiosis in dogs [French]. Pratique Medicale Chirurgicale Anim Compagnie 1990;25:89-97.
32. Martinod S, Gilot B. Epidemiology of canine babesiosis in relation to the activity of Dermacentor reticulatus in southern Jura (France). Exp Appl Acarol 1991;11:215-222.
33. Shakespeare AS. The incidence of canine babesiosis amongst sick dogs presented to the Onderstepoort Veterinary Academic Hospital. J S Afr Vet Assoc 1995;66:247-250.
34. Keller N, Jacobson LS, Nel M, et al. Prevalence and risk factors of hypoglycemia in virulent canine babesiosis. J Vet Intern Med 2004;18:265-270.
35. Birkenheuer AJ, Neel J, Ruslander D, et al. Detection and molecular characterization of a novel large Babesia species in a dog. Vet Parasitol 2004;124:151-160.
36. Kjemtrup AM, Kocan AA, Whitworth L, et al. There are at least three genetically distinct small piroplasms from dogs. Int J Parasitol 2000;30:1501-1505.
37. Zahler M, Rinder H, Zweygarth E, et al. 'Babesia gibsoni' of dogs from North America and Asia belong to different species. Parasitology 2000;120:365-369.
38. Zahler M, Rinder H, Schein E, et al. Detection of a new pathogenic Babesia microti-like species in dogs. Vet Parasitol 2000;89:241-248.
39. Swaminath C. The arthropod vector of Babesia gibsoni. Indian J Med Res 1937;25:499-503.
40. Otsuka H, Yoshimura S. Studies on transmission of Babesia gibsoni Patton (1910) by Haemaphysalis flava Neumann (1897) [Japanese]. Bull Fac Agri Miyazaki Univ 1976;23:511-515.
41. Conrad P, Thomford J, Yamane I, et al. Hemolytic anemia caused by Babesia gibsoni infection in dogs. J Am Vet Med Assoc 1991;199:601-605.
42. Camacho AT, Pallas E, Gestal JJ, et al. Infection of dogs in north-west Spain with a Babesia microti-like agent. Vet Rec 2001;149:552-555.
43. Camacho AT, Guitian EJ, Pallas E, et al. Azotemia and mortality among Babesia microti-like infected dogs. J Vet Intern Med 2004;18:141-146.
44. Camacho AT, Pallas E, Gestal JJ, et al. Ixodes hexagonus is the main candidate as vector of Theileria annae in northwest Spain. Vet Parasitol 2003;112:157-163.
45. Taboada J. Babesiosis. In: Infectious diseases of the dog and cat. 2nd ed. Philadelphia: WB Saunders, 1998; 473-481.
46. Wlosniewski A, Leriche MA, Chavigny C, et al. Asymptomatic carriers of Babesia canis in an enzootic area. Comp Immunol Microbiol Infect Dis 1997;20:75-86.
47. Collett MG. Survey of canine babesiosis in South Africa. J S Afr Vet Assoc 2000;71:180-186.
48. Lobetti RG, Jacobson LS. Renal involvement in dogs with babesiosis. J S Afr Vet Assoc 2001;72:23-28.
49. Yamane I, Conrad P, Gardner I. Babesia gibsoni infections in dogs. J Protozool Res 1993;3:111-125.
50. Yamane I, Gardner IA, Ryan CP, et al. Serosurvey of Babesia canis, Babesia gibsoni and Ehrlichia canis in pound dogs in California, USA. Prev Vet Med 1994;18:293-304.
51. Boozer AL, Macintire DK. Canine babesiosis. Vet Clin North Am Small Anim Pract 2003;33:885-904.
52. Bose R, Jorgensen WK, Dalgliesh RJ, et al. Current state and future trends in the diagnosis of babesiosis. Vet Parasitol 1995;57:61-74.
53. Yamane I, Thomford JW, Gardner IA, et al. Evaluation of the indirect fluorescent antibody test for diagnosis of Babesia gibsoni infections in dogs. Am J Vet Res 1993;54:1579-1584.
54. Fukumoto S, Xuan X, Shigeno S, et al. Development of a polymerase chain reaction method for diagnosing Babesia gibsoni infection in dogs. J Vet Med Sci 2001;63:977-981.
55. Carret C, Walas F, Carcy B, et al. Babesia canis canis, Babesia canis vogeli, Babesia canis rossi: differentiation of the three subspecies by a restriction fragment length polymorphism analysis on amplified small subunit ribosomal RNA genes. J Eukaryot Microbiol 1999;46:298-303.
56. Birkenheuer AJ, Levy MG, Breitschwerdt EB. Development and evaluation of a seminested PCR for detection and differentiation of Babesia gibsoni (Asian genotype) and B. canis DNA in canine blood samples. J Clin Microbiol 2003;41:4172-4177.
57. Uilenberg G, Verdiesen PA, Zwart D. Imidocarb: a chemoprophylactic experiment with Babesia canis. Tijdschr Diergeneeskd 1981;106:118-123.
58. Penzhorn BL, Lewis BD, de Waal DT, et al. Sterilisation of Babesia canis infections by imidocarb alone or in combination with diminazene. J S Afr Vet Assoc 1995;66:157-159.
59. Vercammen F, De Deken R, Maes L. Prophylactic activity of imidocarb against experimental infection with Babesia canis. Vet Parasitol 1996;63:195-198.
60. Tuttle AD, Birkenheuer AJ, Juopperi T, et al. Concurrent bartonellosis and babesiosis in a dog with persistent thrombocytopenia. J Am Vet Med Assoc 2003;223:1306-1310, 1280-1281.
61. Fowler JL, Ruff MD, Fernau RC, et al. Babesia gibsoni: chemotherapy in dogs. Am J Vet Res 1972;33:1109-1114.
62. Wagner JE. Cytauxzoonosis in domestic cats (Felis domestica) in Missouri (abst). J Am Vet Med Assoc 1975;167:874.
63. Bendele RA, Schwartz WL, Jones LP. Cytauxzoonosis-like disease in Texas cats. Southwestern Vet 1976;29:244-246.
64. Ferris DH. A progress report on the status of a new disease of American cats: cytauxzoonosis. Comp Immunol Microbiol Infect Dis 1979;1:269-276.
65. Hauck WN, Snider TG 3rd, Lawrence JE. Cytauxzoonosis in a native Louisiana cat. J Am Vet Med Assoc 1982;180:1472-1474.
66. Glenn BL, Stair EL. Cytauxzoonosis in domestic cats: report of two cases in Oklahoma, with a review and discussion of the disease. J Am Vet Med Assoc 1984;184:822-825.
67. Glenn BL, Kocan AA, Blouin EF. Cytauxzoonosis in bobcats. J Am Vet Med Assoc 1983;183:1155-1158.
68. Kocan AA, Blouin EF, Glenn BL. Hematologic and serum chemical values for free-ranging bobcats, Felis rufus (Schreber), with reference to animals with natural infections of Cytauxzoon felis Kier, 1979. J Wildl Dis 1985;21:190-192.
69. Blouin EF, Kocan AA, Glenn BL, et al. Transmission of Cytauxzoon felis Kier, 1979 from bobcats, Felis rufus (Schreber), to domestic cats by Dermacentor variabilis (Say). J Wildl Dis 1984;20:241-242.
70. Bondy PJ, Cohn LA, Kerl ME. Feline cytauxzoonosis. Compend Cont Ed Pract Vet 2005;27:69-75.
71. Wagner JE, Ferris DH, Kier AB, et al. Experimentally induced cytauxzoonosis-like disease in domestic cats. Vet Parasitol 1980;6:305-311.
72. Ferris DH, Dardiri AH. Experimental infection of domestic animals with a newly discovered American feline protozoan. [Cytauxzoon], in Proceedings. 4th Int Congress Parasitol 1978;71.
73. Cowell RL, Fox JC, Panciera RJ, et al. Detection of anticytauxzoon antibodies in cats infected with a Cytauxzoon organism from bobcats. Vet Parasitol 1988;28:43-52.
74. Greene CE, Latimer K, Hopper E, et al. Administration of diminazene aceturate or imidocarb dipropionate for treatment of cytauxzoonosis in cats. J Am Vet Med Assoc 1999;215:497-500, 482.
75. Chomel BB, Kasten RW, Floyd-Hawkins K, et al. Experimental transmission of Bartonella henselae by the cat flea. J Clin Microbiol 1996;34:1952-1956.
76. Chomel BB, Kasten RW, Sykes JE, et al. Clinical impact of persistent Bartonella bacteremia in humans and animals. Ann N Y Acad Sci 2003;990:267-278.
77. Chomel BB, Boulouis HJ, Breitschwerdt EB. Cat scratch disease and other zoonotic Bartonella infections. J Am Vet Med Assoc 2004;224:1270-1279.
78. Kordick DL, Swaminathan B, Greene CE, et al. Bartonella vinsonii subsp. berkhoffii subsp. nov., isolated from dogs; Bartonella vinsonii subsp. vinsonii; and emended description of Bartonella vinsonii. Int J Syst Bacteriol 1996;46:704-709.
79. Gundi VA, Bourry O, Davous B, et al. Bartonella clarridgeiae and B. henselae in dogs, Gabon. Emerg Infect Dis 2004;10:2261-2262.
80. Mexas AM, Hancock SI, Breitschwerdt EB. Bartonella henselae and Bartonella elizabethae as potential canine pathogens. J Clin Microbiol 2002;40:4670-4674.
81. Chomel BB, Wey AC, Kasten RW. Isolation of Bartonella washoensis from a dog with mitral valve endocarditis. J Clin Microbiol 2003;41:5327-5332.
82. Chomel BB, Mac Donald KA, Kasten RW, et al. Aortic valve endocarditis in a dog due to Bartonella clarridgeiae. J Clin Microbiol 2001;39:3548-3554.
83. Breitschwerdt EB, Blann KR, Stebbins ME, et al. Clinicopathological abnormalities and treatment response in 24 dogs seroreactive to Bartonella vinsonii (berkhoffii) antigens. J Am Anim Hosp Assoc 2004;40:92-101.
84. Kitchell BE, Fan TM, Kordick D, et al. Peliosis hepatis in a dog infected with Bartonella henselae. J Am Vet Med Assoc 2000;216:519-523, 517.
85. Gillespie TN, Washabau RJ, Goldschmidt MH, et al. Detection of Bartonella henselae and Bartonella clarridgeiae DNA in hepatic specimens from two dogs with hepatic disease. J Am Vet Med Assoc 2003;222:47-51, 35.
86. Tsukahara M, Tsuneoka H, Iino H, et al. Bartonella henselae infection from a dog. Lancet 1998;352;1682.
87. Keret D, Giladi M, Kletter Y, et al. Cat-scratch disease osteomyelitis from a dog scratch. J Bone Joint Surg Br 1998;80:766-767.