Treating lymphoma in dogs and cats
Lymphoma is the most common hematopoietic neoplasm affecting both dogs and cats and results from the malignant transformation of lymphocytes.
Lymphoma is the most common hematopoietic neoplasm affecting both dogs and cats and results from the malignant transformation of lymphocytes. Lymphoma often arises from primary and secondary lymphoid tissues, including the thymus, spleen, lymph nodes, and gut-associated lymphoid tissues (Figures 1A, 1B, & 2). However, because lymphocytes are capable of trafficking throughout the body, the development of lymphoma is not anatomically restricted. Common extranodal sites for lymphoma include the skin, eye, central nervous system, testis, and bone marrow (Figures 3 & 4).1
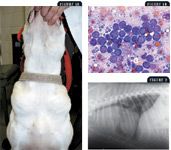
FIGURE 1A. A dog with profound prescapular and mandibular lymphadenopathy. FIGURE 1B. A cytologic preparation obtained from the dog in FIGURE 1A demonstrating a monomorphic population of malignant lymphoblasts, consistent with a clinical diagnosis of lymphoma (Wright's-Giemsa; 400X). FIGURE 2. A lateral thoracic radiograph of a dog with a large cranial mediastinal (thymic) lymphoma.
In dogs, the incidence of lymphoma has been reported to approach 0.1% in susceptible, older individuals, with an annual incidence rate of 84/100,000 dogs at risk.2 Although lymphoma is considered a common neoplasm, a definitive cause for its development in dogs remains to be determined. Several hypothesized causes of canine lymphoma include retroviral infection, environmental contamination with phenoxyacetic acid herbicides, magnetic field exposure, chromosomal abnormalities, and immune dysfunction.3-8 Because lymphoma is a heterogeneous disease process, various causes may be responsible for lymphomas with differing biologic behaviors, as is the case in human non-Hodgkin's lymphoma patients.
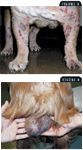
FIGURE 3. Disseminated erythematous dermal plaques in an English bulldog. The histopathologic diagnosis was epitheliotropic T-cell lymphoma (mycosis fungoides). FIGURE 4. An intact male golden retriever with painless bilateral testicular enlargement, cytologically confirmed as a large cell lymphoma.
In cats, feline leukemia virus (FeLV) has been identified as a biologic carcinogen resulting in malignant lymphocyte transformation.9,10 Historical epidemiological investigations before the wide use of preventive FeLV vaccines estimated the annual incidence of feline lymphoma to be 200/100,000 cats at risk.11 With the development of efficacious FeLV vaccines in conjunction with early detection and removal of viremic cats from the general population, the incidence of FeLV-induced lymphoma has been dramatically reduced.12 In addition to FeLV as a causative factor of feline lymphoma, epidemiologic evidence exists for household environmental tobacco smoke to act as a chemical carcinogen, which increases the risk for lymphoma development in cats.13
For most dogs and cats with suspected or confirmed lymphoma, diagnostic evaluations, referred to as clinical staging, should include a complete blood count, a serum chemistry profile, urinalysis, a thoracic radiographic examination, and an abdominal ultrasonographic examination. For patients with anemia, thrombocytopenia, or leukopenia, a bone marrow aspirate or a bone core biopsy should be performed to assess for neoplastic infiltration. Representative cytologic or histologic samples collected from enlarged lymph nodes or other affected organs should be submitted to confirm the diagnosis of lymphoma. Although cytology is a quick and acceptable means for diagnosing lymphoma, histologic evaluation of tissue samples can provide additional prognostic information such as immunophenotype and histologic grade.
Treatment of lymphoma in dogs and cats is often rewarding. However, response to conventional treatment options varies between the two species, as well as among individuals of the same species. To better predict which patients may benefit the most from conventional treatment options, various prognostic factors for both canine and feline lymphoma have been identified. In dogs, clinical substage, histologic grade, immunophenotype, anatomical location, thymidine kinase activity, and histomorphologic subtype appear to influence overall length of survival. In cats, few reliable prognostic factors are recognized, with only initial response to therapy being consistently identified to be predictive of survival time.
Despite the advances made in veterinary oncology over the last decade, systemic chemotherapy remains the cornerstone for treating dogs and cats with lymphoma. In some situations, radiation therapy may be useful when implemented in either an adjuvant or palliative setting. Furthermore, radiation therapy used as a primary treatment modality for solitary extranodal lymphoma, including nasal lymphoma in cats, can provide rewarding and durable clinical responses. Because lymphoma commonly develops in companion animals, veterinary practitioners should be knowledgeable about the treatment options available. Therefore, the focus of this review article is to highlight some of the more conventional therapeutic modalities useful for treating high-grade lymphoma in dogs and cats.
TREATING HIGH-GRADE, MULTICENTRIC CANINE LYMPHOMA
Systemic chemotherapy
Because of the rapid proliferative capacity of malignant lymphocytes, dogs not receiving appropriate treatment for lymphoma often die of terminal tumor burden within several weeks. Thus, it is important that dogs with lymphoma receive prompt and appropriate medical therapy. Systemic chemotherapy remains the treatment of choice for canine high-grade, multicentric lymphoma. Older, yet effective, chemotherapeutic protocols were composed of two distinct treatment phases referred to as induction and maintenance. During the induction phase, dose-intense cytotoxic chemotherapy is administered at frequent intervals over a relatively short time frame, killing most viable cancer cells. After induction chemotherapy, lower dose intensity, long-term chemotherapy called maintenance therapy is instituted to prevent or delay microscopic tumor regrowth. Although maintenance therapy is well-tolerated, it necessitates long-term follow-up treatments, which may be inconvenient and time-consuming for pet owners. Furthermore, it is possible that administering chemotherapy over a prolonged period may ultimately result in cumulative and irreversible bone marrow toxicity. In the past few years, new chemotherapeutic protocols for treating canine high-grade, multicentric lymphoma have excluded the use of maintenance chemotherapy, yet these shorter treatment protocols retain high therapeutic efficacy. Eliminating the requirement for long-term chemotherapy may help pet owners be more receptive to treating their ailing pets with systemic chemotherapy. Although shorter chemotherapeutic protocols provide durable first remission times, you should inform pet owners that most dogs with lymphoma are not cured of their disease. So pet owners should routinely monitor their pets' behavior and activity levels, and you should encourage them to have their dogs evaluated by a veterinarian at least three or four times a year. Through routine health screening, lymphoma relapses may be detected earlier, allowing for the institution of additional chemotherapy before an advanced tumor burden develops.
Most dogs (about 75% to 90%) with high-grade, multicentric lymphoma will achieve complete remission with doxorubicin-based multidrug protocols (Table 1). With numerous chemotherapeutic protocols available, you may find it difficult to decide which therapeutic regimen is best. As a general guideline, you should adopt a protocol that is reliably efficacious but is tolerable even in a compromised patient. Although it is often easier to treat dogs with single-agent oral drug formulations, such as prednisone or lomustine, this practice should be limited to special circumstances in which multiagent protocols cannot be instituted. Multiagent protocols provide superior response rates, disease-free intervals, and survival times when compared with simpler single-agent protocols. Make sure to educate pet owners about the advantages and disadvantages of different treatment regimens.
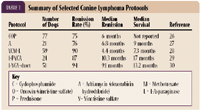
TABLE 1. Summary of Selected Canine Lymphoma Protocols
Relapse
Even with the use of highly effective, multiagent chemotherapeutic protocols, most dogs ultimately relapse. Dogs experiencing lymphoma relapse may achieve long-lasting and clinically relevant second and third remissions with additional treatment. The best combination of chemotherapeutic agents necessary to achieve meaningful second and third remissions depends on several factors including prior drug usage and the amount of time since the patient last received chemotherapy. In many situations, dogs may present with or develop drug-resistant lymphomas that are refractory to traditional chemotherapeutic agents. For these patients, using less conventional antineoplastic drugs or a combination of agents, called rescue therapy, may provide some clinical benefit (Table 2).

TABLE 2. Summary of Selected Rescue Protocols for Canine Lymphoma
Vascular access ports
For systemic chemotherapeutic protocols to be effective, the adequate and consistent delivery of antineoplastic agents is required. Because many dogs are food-motivated, oral chemotherapeutic drugs are generally easy to administer. However, some of the most effective chemotherapeutic agents, such as doxorubicin, are available principally as intravenous formulations. Unlike oral drugs in which bioavailability may be of concern, known quantities of intravenous chemotherapeutic agents can be reliably administered. Although intravenous chemotherapy avoids the unpredictable absorption profiles of oral drugs, some intravenous anticancer agents are irritants or vesicants and can cause marked regional pain and tissue damage when extravasated. Severe tissue necrosis secondary to extravasation of doxorubicin may require surgical débridement and, occasionally, amputation (Figure 5).
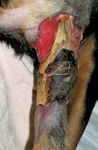
FIGURE 5. Severe tissue necrosis of the antebrachium requiring surgical intervention in a rottweiler two weeks after perivenous extravasation of doxorubicin.
To minimize the likelihood of chemotherapy extravasation, ensure that intravenous catheters are properly placed and firmly secured before chemotherapy administration. In patients in which intravenous catheter placement is difficult, such as markedly obese patients or those with anatomical variations, vascular access ports surgically implanted in the jugular vein may provide a reliable means for repeated intravenous therapy over weeks to months. Vascular access ports are becoming more common in veterinary medicine for the management of both diabetic and cancer patients. Vascular access ports specifically designed for companion animals of various body sizes are available (e.g. Companion Port—Norfolk Vet Products, Skokie, Ill.), and their use should be considered in circumstances in which intravenous access is difficult or when repeated and long-term vascular access is necessary.
Corticosteroids
Corticosteroids cause normal lymphocytes to undergo programmed cell death.14 Similarly, corticosteroids induce cytolytic effects in lymphoid malignancies such as canine lymphoma. Because of their lympholytic properties, corticosteroids such as oral prednisone exert therapeutic effects and can be used to treat canine lymphoma. Given prednisone's low cost, oral formulation, and predictable and familiar side effect profile, veterinary practitioners may be inclined to use single-agent oral prednisone to treat canine lymphoma. About 50% of dogs with high-grade, multicentric lymphoma treated with single-agent prednisone may achieve clinical responses for one to three months. However, the single-agent use of oral prednisone should only be instituted if pet owners, who have been educated of their therapeutic options, actively choose not to pursue superior multiagent chemotherapeutic protocols. Additionally, be aware that the use of oral prednisone before the definitive diagnosis of lymphoma may reduce the efficacy of traditional antineoplastic agents for treating canine lymphoma through the up-regulation of resistance mechanisms.
Multidrug-resistant phenotype is a term used to describe neoplastic cells that are resistant to the cytotoxic effects of certain antineoplastic agents. In dogs with lymphoma, acquired drug resistance has been classically associated with the expression of a drug efflux pump termed P-glycoprotein (Pgp). Functionally, Pgp prevents the retention of cytotoxic agents such as doxorubicin and vincristine within lymphoma cells, allowing malignant lymphocytes to escape lethal DNA injury. Glucocorticoids have been demonstrated to up-regulate Pgp expression in malignant lymphocytes.15 For this reason, treating canine lymphoma with single-agent prednisone should be discouraged, as the net effect may be the promotion of malignant lymphocytes resistant to conventional antineoplastic agents.
Oral lomustine
Lomustine, (CeeNU—Bristol Laboratories) is classified as an antitumor alkylating agent in the nitrosourea family. Lomustine is available in 10-, 40-, and 100-mg capsules. After oral administration, lomustine undergoes hepatic metabolism, and unchanged parent drug and biotransformed metabolites are ultimately eliminated through the kidneys, with biliary excretion and reabsorption from the gut also playing a chief role.16
The safety and efficacy of single-agent lomustine has been evaluated for treating canine lymphoma. Forty-three dogs with lymphoma that had relapsed or had failed to achieve complete remission with previous conventional chemotherapy were treated with single-agent lomustine every 21 days at a dose of 90 to 100 mg/m2. Of the dogs treated, 28% achieved either complete or partial responses for a median duration of 86 days. The principal side effects associated with lomustine administration were hematologic; acute self-limiting neutropenia and cumulative thrombocytopenia occurred in dogs receiving continued long-term lomustine therapy.17
Although hematologic toxicosis was recognized as a self-limiting adverse effect of lomustine therapy in dogs with refractory lymphoma, a more recent study incriminates lomustine as being hepatotoxic in cancer-bearing dogs. In this study, 179 dogs received lomustine for the treatment of various cancer types including lymphoma. After lomustine therapy, hepatotoxicity was identified in a few patients (6.1%).18 Dogs receiving a higher number of doses and higher cumulative doses were more likely to develop hepatotoxicity. Most of the dogs with hepatotoxicity were euthanized as a direct consequence of liver failure. The findings from this study suggest that lomustine, although considered an effective chemotherapeutic agent for treating various cancer types, may induce a delayed, cumulative dose-related, chronic hepatotoxicity that is irreversible and often fatal.
Because single-agent lomustine has demonstrated therapeutic activity for the treatment of refractory canine lymphoma, intuitively, it would be expected that lomustine should possess efficacy when used against drug-naïve, high-grade, multicentric canine lymphoma. Given lomustine's oral formulation, relative low cost, therapeutic effectiveness against refractory lymphomas, and reported low incidence of hepatotoxicity, lomustine appears to be an excellent anticancer drug, but keep in mind that conventional, multiagent systemic chemotherapy is still considered the gold standard for treating canine lymphoma. Carefully consider using single-agent lomustine as a first-line treatment option, and make sure you understand and recognize the potential hazards of using lomustine.
As already demonstrated in dogs treated for refractory lymphoma, the long-term administration of lomustine may result in cumulative thrombocytopenia. When lomustine is used in a rescue setting, lomustine-induced cumulative thrombocytopenia may be of marginal consequence because few effective treatment options remain for heavily treated, relapsing patients. However, in dogs with relapsing lymphoma treated with first-line lomustine, cumulative thrombocytopenia may limit the safe institution of additional myelosuppressive agents, including doxorubicin and cyclophosphamide. In addition to cumulative thrombocytopenia, lomustine's hepatotoxic potential should be carefully considered before its liberal use in cancer-bearing dogs (Figure 6). Although the reported rate of hepatotoxicity is quite low, a recent abstract suggests that the incidence of hepatotoxicity, defined as greater than fourfold elevations in serum alanine transaminase activities, is much higher in dogs treated with a combination of lomustine and oral prednisone. In this study, 23 of 45 dogs treated with lomustine and prednisone had moderately to severely elevated serum alanine transaminase activities.19 Additionally, most dogs (74%) developed pathologic serum alanine transaminase activity elevations with three or fewer lomustine treatments. These findings suggest that combining lomustine with prednisone may markedly increase the likelihood for acute liver injury, and veterinary practitioners should vigilantly monitor patients with serial serum chemistry profiles before each subsequent dose of lomustine, whether alone or in combination with prednisone.
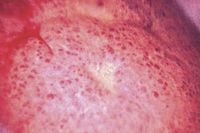
FIGURE 6. A laparoscopic view of the gross morphologic appearance of the liver in a standard poodle with hepatotoxicity attributed to chronic lomustine therapy. Morphologic changes are not specific for lomustine-induced hepatotoxicity.
Lomustine should be considered a viable treatment option for dogs with lymphoma, especially in a rescue setting. However, the recommended use of lomustine as a first-line, single-agent should be reserved primarily for pet owners who actively choose to forgo conventional multiagent chemotherapy for their ailing pets. Currently, no published reports define the efficacy of first-line, single-agent lomustine for the treatment of canine lymphoma. Until that information becomes available, it remains difficult to enthusiastically recommend single-agent lomustine therapy rather than the use of a known effective monotherapy such as doxorubicin.
Adjuvant radiation
Radiation therapy induces programmed cell death in both normal and malignant lymphocytes. Given the sensitivity of malignant lymphocytes to radiation-induced injury, the efficacy of external beam, megavoltage radiation therapy has been evaluated for the adjuvant treatment of high-grade, multicentric lymphoma in dogs. In one study, 52 dogs were treated with a short course of induction chemotherapy (11 weeks), immediately followed by staged, half-body irradiation.20 Radiation therapy was administered to cranial and caudal body halves for a total dose of 8 Gy, given in two fractions of 4 Gy on consecutive days with cobalt-60 photons and a three-week interval between halves. The side effects associated with half-body irradiation were generally mild and included reversible myelosuppression and gastrointestinal upset. In addition to the safety of combined chemotherapy and adjuvant radiation, the investigated protocol was therapeutically effective, with treated patients achieving a first median remission time of 311 days.20 The findings of this study emphasize that long-lasting remissions can be achieved in dogs with lymphoma by using short treatment protocols that combine chemotherapy and radiation.
Nodal irradiation for chemoresistant lymphoma
Malignant lymphocytes expressing multidrug-resistant phenotypes are afforded a survival advantage when exposed to cytotoxic agents. Attempting to treat resistant lymphocyte clones, even with novel chemotherapeutic agents, still may result in disease progression. Because of the limitations of chemical cytotoxic agents, in conjunction with the inherent radiosensitivity of malignant lymphocytes, the use of total lymphoid irradiation (TLI) for confirmed chemoresistant lymphoma has been investigated. In one study, 11 dogs with confirmed multidrug-resistant lymphoma were treated with total nodal irradiation. A dose of 2 Gy given in six fractions over two weeks was administered to all affected peripheral lymph nodes. By the fourth radiation fraction, all treated lymph nodes had returned to normal size, and dogs treated with nodal irradiation survived for a median of 143 days.21 The results of this pilot study demonstrate that dogs with chemoresistant lymphoma may still appreciably benefit from alternative treatment options such as nodal irradiation.
TREATING FELINE LYMPHOMA
Systemic chemotherapy
Unlike canine lymphoma in which several innovative treatment options have been investigated, the treatment of cats with high-grade lymphoma has not markedly changed during the past decade. Several systemic chemotherapeutic protocols have been evaluated for treating feline lymphoma (Table 3). In general, with the institution of multiagent systemic chemotherapy, most (about 60% to 70%) cats will achieve complete remission, with median survival times approximating six to nine months.1 Although the treatment of cats with lymphoma has not changed greatly over the past several years, it has been suggested that cats diagnosed with lymphoma are afforded with better quality-of-life scores and survival times, as compared with a decade ago. In part, this suggestion is based on the dramatic decrease in the number of cats infected with FeLV, a reported negative prognostic factor in cats with lymphoma.22
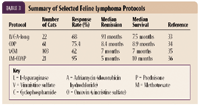
TABLE 3. Summary of Selected Feline Lymphoma Protocols
Radiation therapy for nasal lymphoma
Radiation therapy for regionally confined lymphoma can be rewarding. In cats, lymphoma is the most common nasal tumor (Figure 7). Although large prospective studies are lacking, evidence from a limited number of cats treated for nasal lymphoma with radiation therapy was favorable, with treated cats achieving a median survival time of 19 months.23-25 In our experience, disease progression in cats with nasal lymphoma appears to be more often associated with local regrowth rather than multicentric dissemination. So the benefit of treating feline nasal lymphoma with systemic chemotherapy in addition to curative-intent radiation requires further investigation.
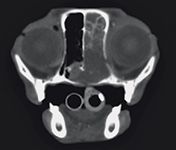
FIGURE 7. A contrast-enhanced transverse computed tomographic image of a cat with a history of chronic stertor, nasal discharge, and decreased appetite. The mass occupies the entire right nasal passage and invades the nasopharynx. Histologic examination of biopsy samples confirmed B-cell lymphoma of the nasal cavity.
CONCLUSION
Lymphoma is a common hematologic malignancy in dogs and cats that usually responds well to therapy. Systemic chemotherapy remains the cornerstone for treating disseminated disease, while radiation therapy may be useful for localized tumor burdens. Most dogs and cats receiving therapy are provided with good quality-of-life scores and prolonged survival times. As newer treatment protocols are investigated and validated for use in companion animals, veterinary practitioners will be able to offer and successfully institute these additional therapeutic options.
Timothy M. Fan, DVM, DACVIM (internal medicine, oncology)
Louis-Philippe de Lorimier, DVM
Department of Veterinary Clinical Medicine
College of Veterinary Medicine
University of Illinois
Urbana, IL 61802
REFERENCES
1. Vail DM, MacEwen EG, Young KM. Canine lymphoma and lymphoid leukemias. In: Withrow SJ, MacEwen EG, eds. Small animal clinical oncology. 3rd Ed. Philadelphia, Pa: WB Saunders, 2001;558-590.
2. Dorn CR, Taylor DO, Schneider R. The epidemiology of canine leukemia and lymphoma. Bibl Haematol 1970;(36):403-415.
3. Chapman AL, Bopp WJ, Brightwell AS, et al. Preliminary report on virus-like particles in canine leukemia and derived cell cultures. Cancer Res 1967;27:18-25.
4. Hahn KA, Richardson RC, Hahn EA, et al. Diagnostic and prognostic importance of chromosomal aberrations identified in 61 dogs with lymphosarcoma. Vet Pathol 1994;31:528-540.
5. Hayes HM, Tarone RE, Cantor KP. On the association between canine malignant lymphoma and opportunity for exposure to 2,4-dichlorophenoxyacetic acid. Environ Res 1995;70:119-125.
6. Hayes HM, Tarone RE, Cantor KP, et al. Case-control study of canine malignant lymphoma: Positive association with dog owner's use of 2,4-dichlorophenoxyacetic acid herbicides. J Natl Cancer Inst 1991;83:1226-1231.
7. Owen LN, Bostock DE, Halliwell REW. Cell-mediated and humoral immunity in dogs with spontaneous lymphosarcoma. Eur J Cancer 1975;11:187-191.
8. Reif JS, Lower KS, Ogilvie GK. Residential exposure to magnetic fields and risk of canine lymphoma. Am J Epidemiol 1995;141:352-359.
9. Francis DP, Cotter SM, Hardy WD Jr, et al. Comparison of virus-positive and virus-negative cases of feline leukemia and lymphoma. Cancer Res 1979;39:3866-3870.
10. Hardy WD Jr, McClelland AJ, Zuckerman EE, et al. Development of virus non-producer lymphosarcomas in pet cats exposed to FeLV. Nature 1980;288:90-92.
11. Essex M, Francis DP. The risk to humans from malignant diseases of their pets: An unsettled issue. J Am Anim Hosp Assoc 1976;12:386-390.
12. Vail DM, Moore AS, Ogilvie GK, et al. Feline lymphoma (145 cases): Proliferation indices, cluster of differentiation 3 immunoreactivity, and their association with prognosis in 90 cats. J Vet Intern Med 1998;12:349-354.
13. Bertone ER, Snyder LA, Moore AS. Environmental tobacco smoke and risk of malignant lymphoma in pet cats. Am J Epidemiol 2002;156:268-273.
14. Bansal N, Houle AG, Melnykovych G. Dexamethasone-induced killing of neoplastic cells of lymphoid derivation: Lack of early calcium involvement. J Cell Physiol 1990;143:105-109.
15. Gruol DJ, Bourgeois S. Expression of the mdr1 P-glycoprotein gene: A mechanism of escape from glucocorticoid-induced apoptosis. Biochem Cell Biol 1994 72:561-571.
16. Oliverio VT. Pharmacology of the nitrosoureas: An overview. Cancer Treat Rep 1976;60:703-707.
17. Moore AS, London CA, Wood CA, et al. Lomustine (CCNU) for the treatment of resistant lymphoma in dogs. J Vet Intern Med 1999;13:395-398.
18. Kristal O, Rassnick KM, Gliatto JM, et al. Hepatotoxicity associated with CCNU (lomustine) chemotherapy in dogs. J Vet Intern Med 2004;18:75-80.
19. Lara A, Kisseberth WC, Couto CG. Hepatotoxicity associated with lomustine treatment in dogs with cancer (abst), in Proceedings. 24th Annu Conf Vet Cancer Soc 2004;14.
20. Williams LE, Johnson JL, Hauck ML, et al. Chemotherapy followed by half-body radiation therapy for canine lymphoma. J Vet Intern Med 2004;18:703-709.
21. Hahn KA. Radiation therapy for drug resistant lymphoma, in Proceedings. 20th Annu Vet Med Forum, 2002;417-418.
22. Vail DM, Moore AS, Ogilvie GK, et al. Feline lymphoma (145 cases): Proliferation indices, cluster of differentiation 3 immunoreactivity, and their association with prognosis in 90 cats. J Vet Intern Med 1998:12:349-354.
23. Straw RC, Withrow SJ, Gillette EL, et al. Use of radiotherapy for the treatment of intranasal tumors in cats: Six cases (1980-1985). J Am Vet Med Assoc 1986;189:927-929.
24. Evans SM, Hendrick M. Radiotherapy of feline nasal tumors. Vet Radiol 1989;30:128-132.
25. Elmslie RE, Ogilvie GK, Gillette EL, et al. Radiotherapy with and without chemotherapy for localized lymphoma in 10 cats. Vet Radiol 1991;32:277-280.
26. Cotter SM, Goldstein MA. Treatment of lymphoma and leukemia with cyclophosphamide, vincristine, and prednisone, I: Treatment of dog. J Am Anim Hosp Assoc 1983;19:159-165.
27. Carter RF, Harris CK, Withrow SJ, et al. Chemotherapy of canine lymphoma with histologic correlation: Doxorubicin alone compared to COP as first treatment regimen. J Am Anim Hosp Assoc 1987;23:587-596.
28. MacEwen EG, Brown NO, Patnaik AK, et al. Cyclic combination chemotherapy of canine lymphosarcoma. J Am Vet Med Assoc 1981;178:1178-1181.
29. Khanna C, Lund EM, Redic KA, et al. Randomized controlled trial of doxorubicin versus dactinomycin in a multiagent protocol for treatment of dogs with malignant lymphoma. J Am Vet Med Assoc 1998;213:985-990.
30. Garrett LD, Thamm DH, Chun R, et al. Evaluation of a 6-month chemotherapy protocol with no maintenance therapy for dogs with lymphoma. J Vet Intern Med 2002;16:704-709.
31. Van Vechten M, Helfand SC, Jeglum KA. Treatment of relapsed canine lymphoma with doxorubicin and dacarbazine. J Vet Intern Med 1990;4:187-191.
32. Rassnick KM, Mauldin GE, Al-Sarraf R, et al. MOPP chemotherapy for treatment of resistant lymphoma in dogs: A retrospective study of 117 cases (1989-2000). J Vet Intern Med 2002;16:576-580.
33. Vail DM, Moore AS, Ogilvie GK, et al. Feline lymphoma (145 cases): Proliferation indices, cluster of differentiation 3 immunoreactivity, and their association with prognosis in 90 cats. J Vet Intern Med 1998;12:349-354.
34. Teske E, van Straten G, van Noort R, et al. Chemotherapy with cyclophosphamide, vincristine, and prednisolone (COP) in cats with malignant lymphoma: New results with an old protocol. J Vet Intern Med 2002;16:179-186.
35. Mooney SC, Hayes AA, MacEwen EG, et al. Treatment and prognostic factors in lymphoma in cats: 103 cases (1977-1981). J Am Vet Med Assoc 1989;194:696-702.
36. Zwahlen CH, Lucroy MD, Kraegel SA, et al. Results of chemotherapy for cats with alimentary malignant lymphoma: 21 cases (1993-1997). J Am Vet Med Assoc 1998;213:1144-1149.