Trilostane: A therapeutic consideration for canine hyperadrenocorticism
A brief review of the diagnosis and treatment of hyperadrenocorticism and the current knowledge on trilostane, including its therapeutic considerations and possible adverse effects.
In 2001, trilostane, a synthetic steroid analogue, was licensed in the United Kingdom for treating canine pituitary-and adrenal-dependent hyperadrenocorticism. Trilostane is currently undergoing Food and Drug Administration (FDA) review for the same purposes in the United States. In this article, I briefly review the diagnosis and treatment of hyperadrenocorticism and then present the current knowledge on trilostane, discuss therapeutic considerations, and address possible adverse effects.
HYPERADRENOCORTICISM
Hyperadrenocorticism is a clinical syndrome arising from chronic, excessive exposure to glucocorticoids. It is also referred to as Cushing's syndrome in recognition of the work done by Dr. Harvey Cushing, a pioneering neurosurgeon, in the early 1900s. There are three types of hyperadrenocorticism. >

Table 1 Clinical Signs of Hyperadrenocorticism
- Pituitary-dependent hyperadrenocorticism (PDH) involves excessive cortisol secretion in response to an inappropriate release of adrenocorticotropic hormone (ACTH) by a pituitary tumor (usually a benign adenoma). This form of hyperadrenocorticism is also called Cushing's disease.
- Adrenal-dependent hyperadrenocorticism involves excessive cortisol secretion by an adrenocortical tumor and can be benign or malignant.
- Iatrogenic hyperadrenocorticism is due to exogenous glucocorticoid administration—oral, parenteral, or topical. It resolves when glucocorticoids are discontinued.
Diagnosis
In patients with the clinical signs of hyperadrenocorticism (Table 1) and supportive findings on routine laboratory tests (Table 2), the diagnosis must be confirmed before therapy is considered.

Table 2 Laboratory Abnormalities Associated with Hyperadrenocorticism
The two screening tests commonly used to diagnose hyperadrenocorticism are the ACTH stimulation test and the low-dose dexamethasone suppression test. The ACTH stimulation test (Table 3)1 is quicker and requires less venipuncture, but it may be less sensitive than the low-dose dexamethasone suppression test is.2 However, it is the only way to identify a patient with iatrogenic hyperadrenocorticism, and it provides information for post-treatment comparisons. The advantage of the low-dose dexamethasone suppression test (Table 4) is its potential for differentiating PDH from adrenocortical tumors.3

Table 3 ACTH Stimulation Testing Methodology and Interpretation
It is important to determine whether a patient has PDH or an adrenocortical tumor. The easiest way to answer this question is by performing an abdominal ultrasonographic examination. A competent scanner can easily identify both adrenal glands and assess their size and shape. Bilaterally normal or enlarged glands support PDH; asymmetry with a mass on one gland and atrophy of the other gland indicates an adrenocortical tumor. Other ways to differentiate between PDH and an adrenocortical tumor include measuring endogenous ACTH concentrations (normal or elevated in patients with PDH, low in patients with hyperadrenocorticism due to an adrenocortical tumor) and performing a high-dose dexamethasone suppression test (suppression supports a diagnosis of PDH). Abdominal radiography may reveal calcification associated with an adrenal mass but is not a sensitive test for this purpose.

Table 4 Low-dose Dexamethasone Suppression Testing Methodology and Interpretation
Treatment
Surgical removal of the affected adrenal gland is the treatment of choice for patients with hyperadrenocorticism caused by an adrenocortical tumor. If the tumor is inoperable, distant metastases are detected, or the patient is an unsuitable anesthetic candidate, medical therapy can be used to control clinical signs.
In the United States, medical therapy is the mainstay of treatment of dogs with PDH. But in people, endoscopic removal of the underlying pituitary tumor is the standard of care and is curative. A successful method for hypophysectomy (rather than tumor removal) has been reported in dogs with hyperadrenocorticism, but it requires substantial expertise and is unlikely to be routinely performed.4
Medical therapy controls the signs of hyperadrenocorticism; it does not cure the disease. Lifelong treatment will be necessary, and owners need to commit to regular follow-up examinations. All of the options have side effects or limitations (Table 5), so provide clients with detailed information before initiating treatment.5

Table 5 Medical Therapy Options for Canine Hyperadrenocorticism
Because of negative experiences or poor responses, some veterinarians are reluctant to recommend treatment in dogs with hyperadrenocorticism. Although studies have not documented improved longevity with therapy, the quality of life for both patients and clients appears to be substantially improved if the disease is successfully controlled. Complications from untreated hyperadrenocorticism include hypertension, diabetes mellitus, glomerulopathy, and thromboembolism; effective regulation of cortisol secretion may protect patients from these debilitating problems.
When selecting a treatment for hyperadrenocorticism, consider likely efficacy, the cost of care (including monitoring), and the risk of adverse events. In general, both ketoconazole and selegiline demonstrate low efficacy and are not widely regarded as appropriate first-line therapies. Choosing between mitotane and trilostane requires careful thought; complications can occur with both drugs, and regular patient evaluations will be necessary. In experienced hands, mitotane is often successful, but its variable intestinal absorption, long half-life, and cytotoxic effects can be problematic. Deciding when to switch from induction to maintenance therapy with mitotane can be difficult, and clients must promptly identify changes in thirst and appetite to prevent overdose. In contrast, trilostane has more predictable pharmacokinetics and is not directly cytotoxic. Daily medication costs may be higher with trilostane, but monitoring expenses may be lower.
TRILOSTANE
Trilostane (4-alpha, 5-alpha-epoxy-17-beta-hydroxy-3-oxoandrostane-2-alpha-carbonitrile) is a synthetic steroid analogue. It is a competitive inhibitor of 3-beta-hydroxysteroid dehydrogenase, an enzyme that catalyses several crucial steps in the synthesis of cortisol from cholesterol (Figure 1).6 When therapeutic concentrations of trilostane are present, cortisol synthesis is dramatically reduced. Although 3-beta-hydroxysteroid dehydrogenase is also required for the synthesis of aldosterone, production of this mineralocorticoid is generally spared at standard therapeutic doses.7 It is thought that the zona glomerulosa (the site of aldosterone production) may be less sensitive to trilostane or that cellular uptake by this region of the adrenal cortex is different.
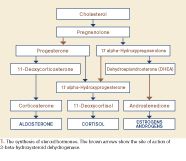
1. The synthesis of steroid hormones. The brown arrows show the site of action of 3-beta-hydroxysteroid dehydrogenase.
Trilostane was previously licensed for use in the United States for people with adrenal disorders but was voluntarily removed from the market in 1994. Work done in the late 1990s in Europe demonstrated trilostane's efficacy in managing canine adrenal disease, and it is presently an approved therapy for dogs with adrenal-and pituitary-dependent hyperadrenocorticism in the United Kingdom and Ireland.
Trilostane is administered orally and appears to be rapidly absorbed. Peak serum concentrations occur one-and-a-half to two hours after dosing and return to baseline within 18 hours; inhibition of steroid synthesis is reported to last less than 20 hours.8 Trilostane undergoes hepatic metabolism, and the pharmacokinetics may be altered in patients with liver dysfunction.
The manufacturers state that trilostane should not be used in patients with primary hepatic disease or renal insufficiency.9 It should be used with caution in anemic patients and avoided in pregnant or nursing bitches or any animal intended for breeding.
Starting therapy
Trilostane is supplied in 10-, 30-, 60-, and 120-mg capsules. The initial dosage is based on body weight (Table 6) and is given once a day with food.9 The dose is then adjusted based on clinical response and ACTH stimulation test results. Most patients show clinical improvement within seven days, with resolution of polydipsia and polyphagia. Re-evaluate all patients, irrespective of clinical status, within the first two weeks. At this time, perform a physical examination, serum chemistry profile including electrolytes, and an ACTH stimulation test. The timing of the ACTH stimulation test is crucial; for the results to be meaningful, it must be started four to six hours after trilostane administration.10

Table 6 Dosage Recommendations for Starting Trilostane Therapy
Monitoring
Dose adjustments are based on the patient's clinical status and post-ACTH stimulation cortisol concentrations. Several different target ranges have been described, but the general consensus suggests that a post-ACTH stimulation cortisol concentration between 1.5 and 5.5 μg/dl indicates optimal control.10-12 According to the U.K. package insert, the acceptable post-ACTH stimulation cortisol concentration range is 1.5 to 9 μg/dl,9 but clinical experience indicates that patients may manifest some signs of hyperadrenocorticism at cortisol concentrations above 5.5 μg/dl.
If the cortisol concentration is below 0.7 μg/dl, withhold trilostane until signs of hyperadrenocorticism recur, and closely monitor the patient for signs of hypocortisolemia.11,13 If the post-ACTH stimulation cortisol concentration is 0.7 to 1.5 μg/dl, suspend therapy for 48 hours, and then restart it with a 50% dose reduction.11,13 If the patient is inadequately controlled, dose increases of 50% to 100% are generally appropriate.11,13 Repeat the clinical evaluation and ACTH stimulation test two weeks after dose adjustment and then every three to six months (Table 7).

Table 7 Monitoring and Dose Adjustment for Dogs Receiving Trilostane
A small number of dogs may have a post-ACTH stimulation cortisol concentration within the optimal range but still show signs of hyperadrenocorticism. In these cases, the dose should be divided and given twice daily and then adjusted based on subsequent ACTH stimulation test results. One recent report indicated that routine twice daily therapy achieved acceptable control of the hyperadrenocorticism with a lower total daily dose, but more work is needed to clarify this issue.14
Response to therapy
More than 85% of dogs have shown both clinical and biochemical improvement (decreased alkaline phosphatase activity and cholesterol concentration) after a month of trilostane therapy, with substantial improvements in post-ACTH stimulation cortisol concentrations.7,11,13 Survival times for patients treated with trilostane (662 to 930 days14,15 ) compare favorably with those receiving mitotane (708 days).15

U.S. importation process of trilostane from the United Kingdom
Adverse effects
Reports indicate that trilostane is generally safe and effective, but complications can occur.11 The most common is transient hypocortisolemia, which manifests as anorexia and lethargy. Clients must be instructed to discontinue trilostane if such signs occur, and an ACTH stimulation test and serum electrolyte panel should be performed. The drug should be restarted with a 50% dose reduction when the patient is again eating and active.
Although trilostane seems to preferentially inhibit cortisol synthesis, aldosterone production can also be compromised.7 If this occurs, serum electrolyte abnormalities are evident (hyponatremia, hyperkalemia), and the patient may appear dehydrated and weak. Once again, discontinuing therapy should be curative, but any patient showing substantial compromise may need fluid support.
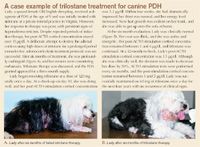
A case example of trilostane treatment for canine PDH
Rarely, trilostane has been associated with acute adrenal gland necrosis.16,17 The mechanism for this is not understood, as the drug is not expected to be cytotoxic. It is possible that complete shutdown of steroid hormone synthesis is somehow injurious to cell metabolism. This rare event does not appear to be dose-dependent because it may occur when therapy is first started or after several months.16,17 It is essential to promptly identify this syndrome and start appropriate treatment (fluid therapy, glucocorticoids, and mineralocorticoids). This complication is permanent and irreversible, and lifelong supplementation of both mineralocorticoids and glucocorticoids will be necessary.
Switching from another therapy
If a patient receiving mitotane, ketoconazole, or selegiline is poorly controlled or adverse effects are noted, a switch to trilostane is appropriate. To minimize complications, I recommend stopping the previous medication for two weeks, so that clinical signs of hyperadrenocorticism are evident before starting trilostane. In addition, an ACTH stimulation test should be done to confirm exaggerated adrenal gland function.
Treating adrenocortical tumors
Historically, functional adrenal tumors are resistant to medical therapy.18 High doses of mitotane may be required to reduce hypercortisolemia, and some patients show no response at all.18 It should be noted, however, that mitotane may have a direct cytotoxic effect on neoplastic adrenal tissue, independent of its ability to effectively control cortisol production.19 Ketoconazole may control clinical signs in up to 30% of dogs, but side effects are commonly reported.20
In contrast, trilostane has been demonstrated to control the clinical signs of hyperadrenocorticism in dogs with adrenocortical tumors, even in dogs with distant metastases.21,22 The drug will not slow tumor growth, but it can control clinical signs and improve patient well-being.21,22
In dogs with operable adrenal tumors, surgical morbidity from infection and thromboembolism may be mitigated by pretreatment with trilostane, although this has not been evaluated systematically.10 I recommend a two-week course at the standard dose, with an ACTH stimulation test performed at day 10. I also recommend that trilostane be discontinued 24 hours before surgery, at which time the usual perioperative management for anticipated hypocortisolemia becomes necessary.
Treating alopecia-X
Alopecia-X is a dermatologic disorder usually described in Pomeranian, poodle, and husky breeds. It is related to an arrest in the normal hair growth cycle and has been associated with deregulation of both growth hormone and adrenal androgen synthesis.23 In classic cases, nonpruritic truncal alopecia occurs; no other signs or changes are noted.
Many of these dogs have elevated concentrations of the precursors to cortisol, particularly 17-hydroxyprogesterone. A recent study evaluating trilostane's effectiveness in 24 affected dogs (Pomeranians and miniature poodles) reported a 90% response rate within eight weeks.24 Trilostane was given once or twice daily, with a mean dose of 10.85 mg/kg/day. No adverse effects were noted, and it was concluded that the hair growth was related to downregulation of adrenal steroid synthesis or inhibition of estrogen receptors within the hair follicles themselves.24 Three affected Alaskan malamutes showed similar positive responses when given 3 mg/kg trilostane twice daily.25
Treating atypical hyperadrenocorticism
Atypical hyperadrenocorticism is a recently described disorder in which patients manifest clinical signs suggesting hyperadrenocorticism, but the diagnosis is not supported by the results of standard screening tests (an ACTH stimulation test and a low-dose dexamethasone suppression test).26 If detailed steroid profiles are performed (e.g. University of Tennessee College of Veterinary Medicine Clinical Endocrinology Service adrenal panel: cortisol, estradiol, androstenedione, 17-hydroxyprogesterone, progesterone, and aldosterone), affected patients have abnormal resting and post-ACTH concentrations of one or more of the cortisol precursors or adrenal androgens or estrogens. Many of these patients have adrenal tumors, so abdominal ultrasonography is always warranted before starting medical therapy.
A detailed discussion of these cases is beyond the scope of this review. However, depending on the particular steroid deregulation identified, trilostane may be an appropriate choice for some of these cases unless a surgical lesion is identified. A thorough understanding of the adrenocortical biochemical pathways is necessary to make an appropriate medical plan (Figure 1), and consultation with an endocrinologist may be helpful. If either mitotane or trilostane is used, careful monitoring for signs of hypocortisolemia is still warranted.
CONCLUSION
Dogs with hyperadrenocorticism that do not respond to mitotane or other forms of medical therapy may benefit from trilostane therapy. Any practitioner using trilostane should be familiar with the likely side effects and be able to adequately monitor patients during therapy. Prompt recognition of potentially life-threatening complications is imperative, and appropriate supportive care must be available. Clients must be informed that trilostane is not approved in the United States, educated on the warning signs of adrenal insufficiency, and given clear instructions about when to discontinue therapy and seek veterinary care.
Editors' note: Dr. Cook is an educational and marketing consultant for Dechra Pharmaceuticals, PLC.
Audrey K. Cook, BVM&S, MRCVS, DACVIM, DECVIM-CA
Department of Small Animal Clinical Sciences
College of Veterinary Medicine & Biomedical Sciences
Texas A&M University
College Station, TX 77843
REFERENCES
1. Behrend EN, Kemppainen RJ, Bruyette DS, et al. Intramuscular administration of a low dose of ACTH for ACTH stimulation testing in dogs. J Am Vet Med Assoc 2006;229(4):528-530.
2. Feldman EC, Nelson RW. Canine hyperadrenocorticism (Cushing's syndrome). In: Canine and feline endocrinology and reproduction. 3rd ed. St. Louis, Mo: Elsevier Saunders, 2004;252-357.
3. Peterson ME. Diagnosis of hyperadrenocorticism in dogs. Clin Tech Small Anim Pract 2007;22(1):2-11.
4. Hanson JM, van 't HM, Voorhout G, et al. Efficacy of transsphenoidal hypophysectomy in treatment of dogs with pituitary-dependent hyperadrenocorticism. J Vet Intern Med 2005;19(5):687-694.
5. Peterson ME. Medical treatment of canine pituitary-dependent hyperadrenocorticism (Cushing's disease). Vet Clin North Am Small Anim Pract 2001;31(5):1005-1014.
6. Potts GO, Creange JE, Hardomg HR, et al. Trilostane, an orally active inhibitor of steroid biosynthesis. Steroids 1978;32(2):257-267.
7. Wenger M, Sieber-Ruckstuhl NS, Müller C, et al. Effect of trilostane on serum concentrations of aldosterone, cortisol, and potassium in dogs with pituitary-dependent hyperadrenocorticism. Am J Vet Res 2004;65(9):1245-1250.
8. Plumb DC. Plumb's veterinary drug handbook. 5th ed. Ames, Iowa: Blackwell Publishing, 2005;778-779.
9. Vetoryl Package Insert. Shrewsbury, Shropshire: Arnolds Veterinary Products/Dechra Veterinary Products.
10. Neiger R, Ramsey IK. Trilostane therapy of canine hyperadrenocorticism, in Proceedings. Am Coll Vet Intern Med Forum 2002.
11. Neiger R, Ramsey I, O'Connor J, et al. Trilostane treatment of 78 dogs with pituitary-dependent hyperadrenocorticism. Vet Rec 2002;150(26):799-804.
12. Reusch CE. New treatment options in canine Cushing's syndrome, in Proceedings. World Small Anim Vet Assoc Congress 2002.
13. Braddock JA, Church DB, Robertson ID, et al. Trilostane treatment in dogs with pituitary-dependent hyperadrenocorticism. Aust Vet J 2003;81(10):600-607.
14. Alenza DP, Arenas C, Lopez ML, et al. Long-term efficacy of trilostane administered twice daily in dogs with pituitary-dependent hyperadrenocorticism. J Am Anim Hosp Assoc 2006;42(4):269-276.
15. Barker EN, Campbell S, Tebb AJ, et al. A comparison of the survival times of dogs treated with mitotane or trilostane for pituitary-dependent hyperadrenocorticism. J Vet Intern Med 2005;19(6):810-815.
16. Chapman PS, Kelly DF, Archer A, et al. Adrenal necrosis in a dog receiving trilostane for the treatment of hyperadrenocorticism. J Small Anim Pract 2004;45(6):307-310.
17. Reusch CE, Sieber-Ruckstuhl N, Wenger M, et al. Histological evaluation of the adrenal glands of seven dogs with hyperadrenocorticism treated with trilostane. Vet Rec 2007;160(7):219-224.
18. Feldman EC, Nelson RW, Feldman MS, et al. Comparison of mitotane treatment for adrenal tumor versus pituitary-dependent hyperadrenocorticism in dogs. J Am Vet Med Assoc 1992;200(11):1642-1647.
19. Plumb DC. Plumb's veterinary drug handbook. 5th ed. Ames, Iowa: Blackwell Publishing, 2005;537-539.
20. Behrend EN, Kemppainen RJ. Medical therapy of canine Cushing's syndrome. Compend Contin Educ Pract Vet 1998;20:679-697.
21. Eastwood JM, Elwood CE, Hurley KJ. Trilostane treatment of a dog with functional adrenocortical neoplasia. J Small Anim Pract 2003;44(3):126-131.
22. Benchekroun G, de Fornel-Thibaud P, Lafarge S, et al. Trilostane therapy of four dogs with metastatic secreting adrenocortical tumor, in Proceedings. Forum Am Coll Vet Intern Med 2007.
23. Frank LA, Hnilica KA, Oliver JW. Adrenal steroid hormone concentrations in dogs with hair cycle arrest (Alopecia X) before and during treatment with melatonin and mitotane. Vet Dermatol 2004;15(5):278-284.
24. Cerundolo R, Lloyd DH, Persechino A, et al. Treatment of canine Alopecia X with trilostane. Vet Dermatol 2004;15(5):285-293.
25. Leone F, Cerundolo R, Vercelli A, et al. The use of trilostane for the treatment of alopecia X in Alaskan malamutes. J Am Anim Hosp Assoc 2005;41(5):336-342.
26. Ristic JME, Ramsey IK, Heath EM, et al. The use of 17-hydroxyprogesterone in the diagnosis of canine hyperadrenocorticism. J Vet Intern Med 2002;16(4):433-439.