An update on three important protozoan parasitic infections in cats: cryptosporidiosis, giardiasis, and tritrichomoniasis
Gastrointestinal parasites are insidious causes of disease in cats. Protozoan parasitic infections in particular can be difficult to detect because there are often no signs of disease, or the signs, such as diarrhea, are nonspecific. But these infections must be uncovered and cured before they cause serious disease or spread to housemates or even owners.
Gastrointestinal parasites are insidious causes of disease in cats. Protozoan parasitic infections in particular can be difficult to detect because there are often no signs of disease, or the signs, such as diarrhea, are nonspecific. But these infections must be uncovered and cured before they cause serious disease or spread to housemates or even owners.
Three important protozoan parasites in cats are Cryptosporidium species, Giardia species, and Tritrichomonas foetus. Cryptosporidium species are coccidians; Giardia species and T. foetus are flagellates. Our understanding of infections with these organisms has changed dramatically in the last several years as the ability to genetically characterize the organisms has advanced. For example, it was previously thought that Giardia and Cryptosporidium species in dogs and cats were identical to those that infect people; it is now known that there are species-specific strains of both organisms. In addition, T. foetus in cats is not zoonotic but was previously thought to be Pentatrichomonas hominis, an agent that also is occasionally detected in the feces of people. There have also been many advances in the diagnosis and treatment of these infections.
In this article, we provide an update on the clinical management of Cryptosporidium species, Giardia species, and T. foetus infections in cats. We emphasize recently published articles and experiences we have had in our research laboratory and clinic.
THE ORGANISMS AND THEIR LIFE STYLES
Cryptosporidium species
In the past, most cases of mammalian cryptosporidiosis were attributed to Cryptosporidiumparvum. However, molecular studies have demonstrated that cats are usually infected with the host-specific Cryptosporidiumfelis.1-5 In one study in our laboratory, all Cryptosporidium species isolated from North American cats were C. felis.5
The life cycle of all Cryptosporidium species begins with a host ingesting sporulated oocysts.6 The 4-x-6-µm oocysts (Figure 1) excyst in the gastrointestinal tract, releasing infective sporozoites, which become enclosed as trophozoites within parasitophorous vacuoles of the microvillus surface of enterocytes. The organisms are covered by the plasma membrane of the host cell, but they do not lie in the cytoplasm. This association with the cytoplasm of the host cell allows it to obtain nutrients.
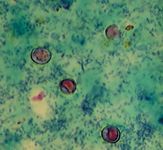
Figure 1. Cryptosporidium species oocysts stained with modified Ziehl-Neelsen acid-fast stain. The oocysts are about 4 x 6 µm.
The trophozoites produce two types of meronts. Within 24 hours, type I meronts leave the parasitophorous vacuoles to invade other epithelial cells where they develop into more type I meronts or type II meronts. Type I meronts can recycle indefinitely, so new type I meronts can arise continuously. The type II meronts produce sexual reproductive stages (gamonts). The zygotes form either thick-walled or thin-walled oocysts, each containing four sporozoites. About 20% of the oocysts produced in the gut are thin-walled oocysts that fail to form an oocyst wall. Thus, Cryptosporidium species appear to have two autoinfective cycles: the first through continuous recycling of type I meronts and the second through sporozoites released from ruptured thin-walled oocysts.7
The thick-walled oocysts are passed in the feces and into the environment. Oocysts are infective on excretion and are extremely environmentally resistant, which permits direct fecal-oral transmission. In one of our studies, after inoculation of cats with C. parvum, C. parvum DNA was detected in the feces on Day 3 after infection, and oocysts were detected on Day 7 after infection.8 In cats, only small numbers of oocysts per gram of feces are passed, making diagnosis difficult.
Giardia species
A Giardia species was first identified in cats by two investigators in 1925; one investigator named it Giardia cati and the other called it Giardia felis.9 It is now known that Giardia duodenalis is a species complex comprising at least eight major assemblages. The feline Giardia (G. cati) is part of the G. duodenalis species complex. Assemblage A of the G. duodenalis species complex has been reported in people, dogs, cats, and other animals. Assemblage B has been found in people and dogs but not in cats.10 Other genetic assemblages within the G. duodenalis group appear to be confined to a specific animal host. Cats have been infected by assemblages known to infect people (assemblage A) as well as assemblages that appear to be specific to cats (assemblage F).
Giardia species occur as trophozoites and cysts. The trophozoite is the active, motile form found in the intestinal tract. It is teardrop-shaped and is about 15 µm long and 8 µm wide. The cyst is the resistant stage mainly responsible for transmission; it is oval and about 12 µm long and 7 µm wide (Figure 2). The cyst can survive several months outside the host in wet, cold conditions but is susceptible to desiccation in dry and hot conditions.
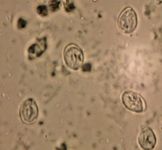
Figure 2. Giardia species cysts after zinc sulfate centrifugation. The cysts are about 8 x 12 µm.
The life cycle is direct, and susceptible hosts become infected by ingesting cysts. The prepatent period of giardiasis in cats ranges from five to 16 days (mean of about 10 days). In contrast to in dogs and people, trophozoites in cats are not found in the duodenum but in the jejunum and ileum.11 Trophozoites multiply by binary fission in the intestinal tract and then encyst by an unknown mechanism. Shedding of Giardia species cysts by cats may fluctuate from undetectable to concentrations of more than 1,000,000 cysts per gram of feces.11 Peaks of cyst shedding occur sporadically rather than cyclically, and the duration between any two given peaks is generally from two to seven days.11 Trophozoites can also be passed in feces but rarely survive for long outside the host.
Tritrichomonas foetus
While cats can also be infected with the commensal flagellate P. hominis, T. foetus infections appear to be more common.12 Isolates of T. foetus from cats are genetically similar to those from cattle; dogs are also occasionally infected.13 The organism is present in a cat's ileum, cecum, and colon as a trophozoite, and it reproduces by binary fission.12 The organism does not encyst, so trophozoites are the only recognized stage (Figure 3). After experimental inoculation in cats, T. foetus was detected in the feces of all cats by Day 14.14

Figure 3. Tritrichomonas foetus trophozoite. The trophozoite is about 10 x 26 µm long by 3 x 15 µm wide. (Photo courtesy of Glenda Taton-Allen, Colorado State University.)
PREVALENCE RATES
Cryptosporidium species
Cats with cryptosporidiosis have been documented worldwide.15,16 However, since genotyping was not performed in most of the studies, the specific prevalence of C. parvum, Cryptosporidium hominis, and C. felis in cats is not exactly known. The results of serologic and fecal testing in prevalence studies depend mainly on the study population and on the diagnostic test used.16
However, serum-antibody-based studies have shown that many cats are exposed. In one seroprevalence study in cats in the United States, about 8% of the cats had been exposed to a Cryptosporidium species.17 Cryptosporidium species oocysts or Cryptosporidium species antigen was detected in feces in 5.4% and 3.8% of the adults cats18 or kittens,19 respectively, in separate studies. When a polymerase chain reaction (PCR) assay was used to amplify Cryptosporidium species DNA in the feces of cats with diarrhea, 29.4% of the 180 cats tested had positive results.20
Giardia species
Giardia species infections in cats have also been reported around the world. And like cryptosporidiosis, reports of giardiasis prevalence rates have varied based on the population tested and the diagnostic test used. For example, in one study in adult cats in north-central Colorado, the prevalence of giardiasis was 3.9% and 1.9% in cats with and without diarrhea, respectively.18 In a study of kittens less than 1 year of age residing in New York state, Giardia species was detected in 6.1% and 8.1% of client-owned and shelter cats, respectively.19
Tritrichomonas foetus
The worldwide prevalence rate of T. foetus infection in cats is unknown. Infections appear to be most common in cats in crowded environments; infection in feral cats and healthy cats is uncommon. In one study in cats at an international cat show, 31% of 117 cats owned by 89 different breeders were infected.21

Client discussion points
TRANSMISSION
Cryptosporidium species, Giardia species, and T. foetus can be transmitted by ingesting feces from mutual grooming or shared litter boxes. For Giardia and Cryptosporidium species, ingestion of contaminated food or water and contact with other infected animals (i.e. ingestion of prey species) is also likely to be associated with transmission.22 Although infection in cats with these agents is common, many infected cats have no clinical signs. Diarrhea is generally more common in young animals.9,23,24 Coinfection with more than one agent has also been reported.
PATHOGENESIS
Cryptosporidium species
When it occurs, diarrhea caused by cryptosporidiosis is associated with impaired intestinal absorption and enhanced anion secretion.25,26 Histologic examination of small-intestinal lesions from infected cats reveals loss of microvilli, degeneration of host epithelial cells, and atrophy of the villi.27 In some naturally infected cats, mild to moderate lymphocytic-plasmacytic duodenitis has been detected; however, it is unknown whether the inflammation was preexisting or from Cryptosporidium infection.27-29 Mononuclear cell infiltrates in the duodenum are common in AIDS patients with cryptosporidiosis, and as in some cats, the infiltrates resolve in some patients after treatment, suggesting the organism was the cause of the inflammation.30
It is not known why some cats and not others develop clinical signs of disease. The development of clinical signs might depend on various factors including the presence of immunodeficiency, coinfections, other gastrointestinal diseases, host susceptibility, or infection with more-pathogenic strains.
Giardia species
Giardia species cysts excyst in the duodenum after exposure to gastric acid and pancreatic enzymes and release two trophozoites that then separate, mature, and attach to the brush border of the villous epithelium throughout the intestinal tract in cats. The pathogenesis of diarrhea may be related to secretory-excretory products of the parasite. As with Cryptosporidium species, clinical signs may be related to immunodeficiency, coinfections, other gastrointestinal diseases, host susceptibility, or infection with more-pathogenic strains.
Tritrichomonas foetus
Several elements, such as adhesion, secretion of proteases, and release of lytic factors, are involved in the mechanism of tissue damage of T. foetus.31 In a study of naturally infected cats, mild-to-moderate lymphocytic-plasmacytic and neutrophilic colitis and crypt epithelial cell hypertrophy were common.32 Coinfection with other organisms occurs and influences the pathogenesis of disease. For example, cats with preexisting Cryptosporidium species infection had more severe signs of disease when experimentally inoculated with T. foetus than did cats inoculated with T. foetus alone.12 However, coinfections with Cryptosporidium species and T. foetus are rare in naturally exposed cats. Other predisposing factors as described for Cryptosporidium and Giardia species may also play a role in the pathogenesis of this disease.
CLINICAL SIGNS
Cryptosporidium species
Most cats with Cryptosporidium species infection have subclinical disease.15,33,34 Some infected cats develop small bowel diarrhea, weight loss, and anorexia. Clinical signs associated with infection can occur in immunocompetent or immunodeficient cats.28,35,36 Coinfection with other protozoans including Giardia species and T. foetus may aggravate clinical signs of cryptosporidiosis in cats.13,37,38
Giardia species
Although most of the cats shedding Giardia species do not show clinical signs of disease, some develop acute or chronic diarrhea and weight loss. The diarrhea is usually mucoid, pale, and soft and has a strong odor; steatorrhea may be present.9,11,39,40 Most infected cats are afebrile, do not vomit, and have normal total protein and hemogram values.9
Tritrichomonas foetus
Kittens with T. foetus-associated illness usually present for evaluation of chronic large bowel diarrhea. The diarrhea is often semiformed and malodorous and contains blood or mucus. The anal area frequently becomes edematous, and feces can fall from the anus. Clinical signs of disease are often intermittent and usually resolve with antimicrobial therapy, only to recur after therapy is discontinued.
DIAGNOSIS
Because there are so many infectious causes of diarrhea in cats, the American Association of Feline Practitioners (AAFP) Zoonoses Guidelines committee recommends that a fecal wet mount, fecal cytology, fecal flotation, and Cryptosporidium screening procedure be performed as the minimum diagnostic plan in all cats with diarrhea.41 Here are the common methods used to diagnose Cryptosporidium species, Giardia species, and T. foetus infections in cats.
Cryptosporidium species
In cats, few Cryptosporidium species oocysts per gram of feces are shed. The small numbers of oocysts combined with the small size of oocysts result in failure to diagnose the infection with microscopic examination of feces after routine fecal flotation. Staining a thin fecal smear with modified Ziehl-Neelsen acid-fast stain can help demonstrate Cryptosporidium species oocysts (Figure 1). The oocysts are pink to bright-red spheres about 4 to 6 µm in diameter.
A fluorescein-labeled monoclonal antibody-based assay (IFA) that can simultaneously detect Cryptosporidium and Giardia species (Merifluor Cryptosporidium/Giardia—Meridian Bioscience) is available. This test was originally developed for use in human samples, so some infections in cats may not been detected. When a single feline fecal sample was tested, the sensitivity of the IFA was lower than that of the modified Ziehl-Neelsen staining technique.42 However, the sensitivity of the IFA equals that of modified Ziehl-Neelsen acid-fast staining when two to four feline fecal samples collected on consecutive days are tested.42 False negative results can occur with both tests. The same IFA was reported to be more sensitive and specific than zinc sulfate flotation and was comparable to an antigen detection technique when used on refrigerated feline fecal samples.43 Experiments in our laboratory suggest this assay may be the best for secondary screening of cat feces for Cryptosporidium species infections if the results of modified Ziehl-Neelsen acid-fast staining are negative (Lappin MR, Department of Clinical Sciences, College of Veterinary Medicine and Biomedical Sciences, Colorado State University, Fort Collins, Colo: Unpublished data, 2006). However, the assay requires a fluorescence microscope, so most practices must send samples to a clinical pathology laboratory.
A number of point-of-care C. parvum antigen tests are available for use with human feces. The results of these tests with feline feces have been variable.42,43 We recently evaluated two lateral flow devices marketed for the detection of C. parvum in human feces and found them to be inadequate for detecting Cryptosporidium species in feline feces (Bachman D, Lappin MR, Department of Clinical Sciences, College of Veterinary Medicine and Biomedical Sciences, Colorado State University, Fort Collins, Colo: Unpublished data, 2006). This may relate to antigenic differences between C. felis and C. parvum; currently available point-of-care kits use monoclonal antibodies directed against C. parvum.
We optimized a PCR assay used to amplify Cryptosporidium species DNA in feline feces and showed it to be more sensitive than IFA.8 However, positive test results only document infection, not clinical illness due to infection. In general, reserve the use of Cryptosporidium species PCR assays for cats with chronic, unexplained diarrhea that have negative results on other tests. DNA sequencing can be used to differentiate Cryptosporidium and Giardia species.1,5,44
Giardia species
Evaluate the feces of all cats with diarrhea for Giardia species and T. foetus trophozoites. Mix a small amount of mucus or feces collected by loop or from the surface of freshly passed diarrhea with a drop of 0.9% saline solution on a microscope slide, cover it with a coverslip, and examine it immediately. A refrigerated sample or one examined several hours after collection probably contains no living organisms. At 100×, the only visible motility may be the flagella.45 Giardia species trophozoites have a falling leaf motility pattern in contrast to the rapid, jerky, forward motion of T. foetus. Structural characteristics such as the concave ventral disk can be observed at 400×. Applying Lugol's iodine solution, methylene blue, or acid methyl green to the wet mount helps you see the internal structures of the trophozoites.
As stated above, all cats with diarrhea should have fecal flotation performed. The chances of detecting protozoal cysts are thought to be greatest after using fecal concentration techniques that involve centrifugation, such as sugar centrifugation and zinc sulfate centrifugation.46 Technical information on performing these tests is on the Companion Animal Parasite Council (CAPC) Web site (www.capcvet.org). Because sugar solution is hypertonic and pulls the cytoplasm of the cysts to one side, making it appear as a half or quarter moon, some parasitologists prefer zinc sulfate solution. After concentration in any solution, the microscope slide should be read within 15 to 20 minutes after being prepared because eventually the cysts will collapse. The feces can be refrigerated, but not frozen, if there is a delay before testing.
The 8-x-12-µm and 7-x-10-µm cysts (Figure 2) can be can be confused with yeast, but Giardia species cysts should be easily recognized because of their distinct structure. At 100×, the cysts are about the size of a neutrophil. At 400× and greater, internal structures such as nuclei can be detected (Figure 2). Because of the intermittent pattern of shedding, examine at least three samples obtained over a period of about one week before ruling out giardiasis. This frequency of examination gave a sensitivity of about 90% in one study in dogs that involved the zinc sulfate centrifugation technique.47
We have used an IFA for simultaneous detection of Cryptosporidium and Giardia species (Merifluor Cryptosporidium/Giardia) in several of our studies.8,37,43 In our opinion, this assay reliably aids in the detection of Giardia species cysts in cat feces. We think the assay results are superior to those of antigen tests because the test is unlikely to give false positive results; not only are positive samples fluorescing, but the cysts can also be measured to make sure they are morphologically consistent with Giardia species. However, the assay requires a fluorescence microscope.
Enzyme-linked immunosorbent assays (ELISAs) titrated for use with human feces have been used to detect Giardia species antigen in dog and cat feces in several studies with variable results.45,48,49 Recently, a point-of-care Giardia species antigen test for use with dog or cat feces (Snap Giardia—IDEXX Laboratories) was introduced. Results of the assay compared favorably with IFA in studies completed by the company.45 In a study recently completed in our laboratory, results of this ELISA and IFA were in agreement for 94.4% of the samples (Bachman D, Lappin MR, Department of Clinical Sciences, College of Veterinary Medicine and Biomedical Sciences, Colorado State University, Fort Collins, Colo: Unpublished data, 2006). If the results of either a wet mount examination or fecal flotation are positive, a fecal antigen test is not needed except as a confirmation test in questionable samples. It is our opinion that if Giardia species antigen testing is considered, the Snap Giardia assay is probably the most logical to use. However, the assay should be a supplemental test and should not replace fecal flotation and wet mount examination.
PCR assays can also be used to amplify Giardia species DNA in feces and are available in some research laboratories. However, we think that a direct smear, the immunologic tests (IFA, antigen tests), and PCR assays should only be used in cats with diarrhea (see "Zoonotic concerns and prevention in people" below).
Tritrichomonas foetus
Perform a direct smear on diarrhea samples from all cats with large bowel diarrhea to detect T. foetus trophozoites by using the technique described for Giardia species. This organism is similar in size to Giardia species but can be differentiated by an undulating membrane, rapid forward motion, the lack of a concave surface, and a single nucleus. The sensitivity of a direct smear using samples from naturally infected cats was only 14% in one study, so results can be falsely negative.50
If you still suspect T. foetus infection after the initial workup, a culture can be performed at a diagnostic laboratory or in-house by using a commercially available culture system (InPouch TF—BioMed Diagnostics). The medium used does not support the growth of Giardia species or P. hominis, so positive test results are likely to correlate to infection by T. foetus.50 A PCR assay is also commercially available.51 Information concerning culture and PCR assay detection of T. foetus infection is available at www.cvm.ncsu.edu/mbs/gookin_jody.htm.
TREATMENT
Because Cryptosporidium species, Giardia species, and T. foetus have been detected in the feces of cats with and without diarrhea, a positive test result does not always prove the agent is the cause of diarrhea. If the diagnostic workup does not reveal another cause of diarrhea, initiate treatment. Like bacteria, protozoans can have variable responses to different drugs. So be prepared to try an alternate drug if a cat's clinical signs don't resolve.
Cryptosporidium species
More than 100 compounds have been evaluated to treat cryptosporidiosis in laboratory animal models, people, and cattle, but no treatment has consistently eliminated clinical signs or the organism from the gastrointestinal tract. Few studies have described the treatment of feline cryptosporidiosis, and to our knowledge, none have been controlled.
Tylosin
In one case report, clindamycin hydrochloride (25 mg/kg orally daily) was administered to a cat with chronic cryptosporidiosis and lymphocytic duodenitis.28 After 60 days of therapy, there was no further improvement in stool consistency, and oocysts were still detected. So clindamycin was discontinued on Day 60, and tylosin was administered (11 mg/kg orally twice daily) for 28 days. Stools were normal within seven days after tylosin therapy was initiated, and the fecal samples assessed for oocysts during the treatment were negative. The results from eight fecal examinations were negative for oocysts six months after the completion of tylosin therapy. The inflammatory changes in the bowel resolved after treatment, suggesting the inflammation was from Cryptosporidium species infection.
We have treated many cats with presumed cryptosporidiosis with tylosin at a dosage of 10 to 15 mg/kg given orally twice daily for 21 days, and diarrhea has resolved in about 50% of the cases (Lappin MR, Scorza AV, Department of Clinical Sciences, College of Veterinary Medicine and Biomedical Sciences, Colorado State University, Fort Collins, Colo: Unpublished data, 2006). However, these observations are uncontrolled, and the signs in the affected cats may have resolved spontaneously. It is also possible the anti-inflammatory or antibacterial effects of tylosin played a role in clinical responses. In addition, tylosin is not tolerated by most cats because of its unpleasant taste.
Azithromycin
Azithromycin has been evaluated in animal models of infection and in people that have cryptosporidiosis with some encouraging preliminary results.52 Azithromycin achieves high biliary concentrations. In a recent study, administering azithromycin to dairy cows infected with Cryptosporidium species significantly reduced oocyst shedding and improved clinical signs of diarrhea.53 We currently recommend azithromycin at a dosage of 10 mg/kg given orally daily in cats that are intolerant or nonresponsive to tylosin. The optimal duration of therapy is unknown but is usually several weeks. Other than the potential for mild gastrointestinal side effects, the drug appears safe for use in cats.
Paromomycin
Paromomycin is an aminoglycoside antibiotic that is poorly absorbed from the normal gastrointestinal tract. The efficacy of the drug in people that have AIDS and cryptosporidiosis is controversial. However, in clinical practice, paromomycin is commonly used to treat cryptosporidiosis in patients with HIV infection.54 Paromomycin was effective in clearing Cryptosporidium species oocysts from the feces of a naturally infected cat with persistent diarrhea55 and from the feces of eight experimentally inoculated cats (McReynolds C, McReynolds LM, Brewer MM, et al. Master's defense, Colorado State University, Fort Collins, Colo: Unpublished data, 2006). However, the diagnostic tests used in the follow-up period in both studies may not have been sensitive enough to detect animals in a carrier state.
We have recently studied groups of presumably immunocompetent kittens and adult cats that were infected with both Giardia and Cryptosporidium species (Scorza AV, Lappin MR, Department of Clinical Sciences, College of Veterinary Medicine and Biomedical Sciences, Colorado State University, Fort Collins, Colo: Unpublished data, 2006). Cats with coinfections seem to be more difficult to treat than cats infected with either organism alone. Recently, we used paromomycin at a dosage of 150 mg/kg given orally daily to control diarrhea in two cats with resistant Giardia species and C. felis infection that had not responded to treatment with other drugs. However, treatment was needed for more than 21 days to achieve maximal clinical response and stop fecal shedding.
Paromomycin should be considered a rescue drug (to be used only in resistant infections) and should never be used in cats with bloody diarrhea because of the risk of systemic absorption and induction of renal disease or deafness. In one study of cats with Tritrichomonas species infection treated with paromomycin (125 to 165 mg/kg orally every 12 hours for five days), four out of 32 cats developed acute renal failure, and three of the four cats became deaf.56
Nitazoxanide
In people, one of the most promising agents used to treat cryptosporidiosis is nitazoxanide, a derivative of nitrothiazole, which is effective against a broad spectrum of parasites and bacteria. To date, nitazoxanide is the only drug approved by the FDA to treat diarrhea caused by Cryptosporidium and Giardia species in children.57,58 We are currently evaluating nitazoxanide to treat a number of small-animal parasites. Some of the dogs and cats with Cryptosporidium or Giardia species infections have had diarrhea resolve after the administration of nitazoxanide at a dosage of 25 mg/kg given orally every 12 hours for at least five days, but it is too early to suggest the efficacy of this treatment.
Giardia species
Because of the potential for zoonotic transmission, the treatment of cats with giardiasis may be advocated whether or not they are clinically ill. In human medicine, a combination of nutritional intervention and phytotherapy is the first line of approach for treating giardiasis because the infections are often self-limiting and drug therapy likely does not eliminate infection. Antibiotic treatment is often reserved for cases in which the nutritional approach has been ineffective.
Nutritional intervention
Nutritional management is based on foods and supplements that inhibit Giardia species growth, replication, or attachment to the enterocytes and that promote host immune defense against the parasite. Consuming a whole-food, high-fiber, low-simple-carbohydrate, low-fat diet will help reduce the acute signs of giardiasis in people.59 Probiotics, wheat germ, and medicinal herbs including garlic and flavonoid-containing herbs have been reported to have anti-Giardia species properties.59 However, the efficacy of these approaches for treating giardiasis in cats is unknown. We generally use highly digestible bland diets if vomiting and small bowel diarrhea are the main clinical signs. If large bowel diarrhea is the principal clinical sign, high-fiber diets are used.
Drug therapy
While dietary manipulation is often used, most veterinary clinicians also prescribe drugs. In the last several years, several studies have been published concerning the treatment of feline giardiasis; the following are the protocols recommended by our laboratory.
In several studies, metronidazole effectively controlled diarrhea and eliminated Giardia species cyst shedding in naturally and experimentally infected cats.40,60-62 Metronidazole may also help correct secondary bacterial overgrowth. Thus, if fecal cytology is consistent with concurrent bacterial disease, we generally prescribe metronidazole at the maximal dosage of 25 mg/kg given every 12 hours for seven days.62 If the owner can afford the formulation fee, we use metronidazole benzoate in tuna flavor.62 The USP formulation is routinely available in the United States but induces salivation and inappetence when administered in some cats.63 The protozoal toxicity of metronidazole is from short-lived intermediates or free radicals that produce damage by interacting with DNA and possibly other molecules.64 Gastrointestinal or central nervous system toxicosis has been associated with metronidazole administration in some kittens at either an excessive dose or from a probable cumulative neurotoxic effect.65
Benzimidazoles may have an anti-Giardia species effect by interacting with the colchicine site in tubulin in the microtubules, resulting in the disruption of assembly and disassembly.66 Selective toxicity is achieved because the drug is minimally absorbed from the host intestine.66 If the clinical history and laboratory findings are most consistent with concurrent Giardia species and nematode infections, we generally prescribe fenbendazole at a dosage of 50 mg/kg given orally every 24 hours for at least five days. Fenbendazole was shown to be safe when administered to healthy, adult, nonpregnant cats at a dose five times higher than the approved dose in wild felids and dogs.67 The suggested dosage for fenbendazole for the treatment of giardiasis is 50 mg/kg given orally every 24 hours for three to five days.68 However, when fenbendazole was administered in cats concurrently infected with Giardia species and C. parvum, only four of eight cats stopped shedding Giardia species cysts.37 Albendazole has been used successfully to treat giardiasis in dog studies but can cause bone marrow suppression in cats and dogs. Thus, we do not currently prescribe it to cats or dogs.69
Febantel is a benzimidazole found in a combination product containing febantel, pyrantel, and praziquantel (Drontal Plus—Bayer Animal Health). When we administered the product to adult cats empirically at a dosage of two small dog tablets per cat (about 50 mg/kg febantel) orally for five days, decreases in cyst shedding by experimentally infected cats were noted.70 Additionally, four of the six treated cats had no evidence of cysts even after the administration of glucocorticoids in an attempt to induce immunosuppression. Febantel is, in part, metabolized to fenbendazole, which likely explains its benefit for giardiasis.71 Whether this drug is superior to fenbendazole is unknown.
We have prescribed paromomycin or nitazoxanide in some cats with naturally occurring, resistant giardiasis by using the protocols described for cryptosporidiosis. However, none of the data are controlled, so whether these drugs are reasonable first-choice drugs is debatable.
Vaccination
The Giardia species vaccine used as a preventive in dogs was apparently successful in eliminating cyst shedding and diarrhea in a group of naturally infected dogs.72 However, when we infected 16 cats with Giardia species and then administered three doses of a commercially available feline Giardia species vaccine (Fel-O-Vax Giardia—Fort Dodge Animal Health) to eight kittens, we could not detect a difference in cyst shedding between vaccinates and controls, suggesting that the vaccine was an ineffective therapy.73 However, only one Giardia isolate was used, so whether the vaccine is an effective immunotherapy in naturally infected cats is unknown.
Treatment failures
Treatment failures are common in people and other animals with giardiasis. It is likely that no drug will be universally effective for treating giardiasis. So in clinical practice, vary the drug and protocol you use according to each individual patient, and consider all other options for treatment. In chronic cases, also consider the possibility of underlying disorders such as inflammatory bowel disease, bacterial overgrowth, exocrine pancreatic insufficiency, and immunodeficiency. Infection with Giardia species does not appear to cause permanent immunity, so reinfection can occur, hampering the assessment of treatment studies.
Tritrichomonas foetus
Multiple treatment regimens have been attempted in cats with T. foetus infections with generally poor responses. In some cases, temporary improvement was noted with a variety of drugs, but relapse was invariable. Recently, ronidazole was shown to have in vitro and in vivo activity against one strain of T. foetus in cats and, for now, should be considered the drug of choice.14 Administer ronidazole at a dosage of 30 mg/kg orally every 12 hours for 14 days. The drug has to be formulated for use (Westlab Pharmacy, Gainesville, Fla.).
FOLLOW-UP TESTING
Optimal follow-up testing recommendations for Cryptosporidium species, Giardia species, and T. foetus infections have been difficult to make because 1) even in cats with negative test results, it is unknown whether infection still exists below the sensitivity limit of the assay used, and 2) none of the infections result in permanent immunity, so infection can be quickly reacquired from the environment or other infected cats. Thus, it is our opinion that the primary goal of therapy is to eliminate clinical signs of disease.
The AAFP Zoonoses Guidelines committee recommends at least one follow-up examination for animals with previous diagnosis of an enteric zoonotic agent.41 Assuming the diarrhea has resolved, perform a fecal flotation for Giardia species cysts and Cryptosporidium species oocysts within nine days. If the results are negative, perform testing by fecal flotation at least once or twice a year as part of a routine health check. Cats without diarrhea in general are not considered human health risks.
PREVENTING INFECTION IN PETS
It is extremely difficult to prevent cats from being exposed to Giardia species, Cryptosporidium species, or T. foetus because the organisms are transmitted by fecal-oral contact and reinfection can occur. Attempts can be made to avoid contact with infected animals; however, shedding can be intermittent, and treatment does not eliminate infection in most animals. Tritrichomonas foetus does not have an environmentally resistant stage. But Giardia species cysts and Cryptosporidium species oocysts can exist outside the host. While Giardia species cysts can be killed on surfaces after one minute of contact time with quaternary ammonium compounds, Cryptosporidium species oocysts are resistant to routine disinfectants.74 Only extreme temperatures affect the viability of the oocysts: Exposing the oocysts to 139.5 F (59.7 C) for five minutes, freezing the oocysts to -94 F (-70 C), and desiccating the oocysts for four hours kills the oocysts.74
If giardiasis is recurring in a cattery, shelter, or multicat household, control measures could include
1. Cleaning and decontaminating the environment
2. Administering drugs with anti-Giardia species effects to all animals
3. Cleaning cysts from coats
4. Preventing reinfection.75
Animals can be bathed with regular pet shampoos and thoroughly rinsed. The use of Giardia species vaccines as preventives in cats is still controversial because it is unknown whether the vaccine induces protection against feline-specific Giardia species isolates. In dogs, administering the vaccine in the field did not change the incidence of giardiasis in several studies.48,76
ZOONOTIC CONCERNS AND PREVENTION IN PEOPLE
Tritrichomonas foetus infection is not zoonotic. Most cases of cryptosporidiosis in people are caused by the C. parvum cattle genotype and C. hominis; most cats are infected with C. felis. However, C. felis DNA has been amplified from both immunocompetent and immunosuppressed people.1-3 An epidemiological study failed to find an association between pet ownership and cryptosporidiosis in people with HIV infection.77 Similarly, Giardia species isolates from people and cats often vary, and to our knowledge, transmission of Giardia species from a cat to a person has not been documented. However, all cats infected with either Giardia or Cryptosporidium species should be considered potentially zoonotic.
People can attempt to avoid Giardia and Cryptosporidium species infections by avoiding contaminated food or water and by disinfecting contaminated areas. Water collected in the wilderness or rural areas should be filtered or boiled before drinking. The Centers for Disease Control and Prevention's online publication recommends using filters labeled as reverse osmosis and others with a pore size of 1 micron or less.78 Chlorination of drinking water is not sufficient to kill Cryptosporidium species oocysts.
Andrea V. Scorza, MV, MS
Michaell R. Lappin, DVM, PhD, DACVIM
Department of Clinical Sciences
College of Veterinary Medicine and Biomedical Sciences
Colorado State University
Fort Collins, CO 80523
REFERENCES
1. Xiao L, Bern C, Limor J, et al. Identification of 5 types of Cryptosporidium parasites in children in Lima, Peru. J Infect Dis 2001;183:492-497.
2. Pedraza-Diaz S, Amar C, Iversen AM, et al. Unusual Cryptosporidium species recovered from human faeces: first description of Cryptosporidium felis and Cryptosporidium 'dog type' from patients in England. J Med Microbiol 2001;50:293-296.
3. Morgan UM, Weber R, Xiao L, et al. Molecular characterization of Cryptosporidium isolates obtained from human immunodeficiency virus-infected individuals living in Switzerland, Kenya, and the United States. J Clin Microbiol 2000;38:1180-1183.
4. Pieniazek NJ, Bornay-Llinares FJ, Slemenda SB, et al. New cryptosporidium genotypes in HIV-infected persons. Emerg Infect Dis 1999;5:444-449.
5. Scorza AV, Burnett R, Black WC IV, et al. Molecular and phylogenetic characterization of Cryptosporidium from cats and dogs (abst), in Proceedings. Int GiardiaCryptosporidium Conf 2004.
6. Marquardt WC, Demaree RS, Grieve RB. The intestinal coccidian. In: Parasitology and vector biology. 2nd ed. San Diego, Calif: Hartcourt/Academic Press, 2000;29-35.
7. Parasitology Research, Division of Biology, Kansas State University. Basic biology of Cryptosporidium. Available at: www.k-state.edu/parasitology/basicbio. Accessed Feb 21, 2006.
8. Scorza AV, Brewer MM, Lappin MR. Polymerase chain reaction for the detection of Cryptosporidium spp. in cat feces. J Parasitol 2003;89:423-426.
9. Kirkpatrick CE. Feline giardiasis: a review. J Small Anim Pract 1986;27:69-80.
10. Monis PT, Thompson RCA. Cryptosporidium and Giardia-zoonoses: fact or fiction? Infect Genet Evol 2003;3:233-244.
11. Kirkpatrick CE, Farrell JP. Feline giardiasis: observations on natural and induced infections. Am J Vet Res 1984;45:2182-2188.
12. Gookin JL, Levy MR, Law JM, et al. Experimental infection of cats with Tritrichomonas foetus. Am J Vet Res 2001;62:1690-1697.
13. Gookin JL, Birkenheuer AJ, St. John V, et al. Molecular characterization of trichomonads from feces of dogs with diarrhea (abst), in Proceedings. Am Coll Vet Intern Med 2005.
14. Gookin JL, Copple C, Papich M, et al. Efficacy of ronidazole in vitro and in vivo for treatment of feline Tritrichomonas foetus infection (abst), in Proceedings. Am Coll Vet Intern Med 2005.
15. Iseki M. Cryptosporidium felis sp.n. (Protozoa: Eimeririna) from the domestic cat. Jpn J Parasitol 1979;5:285-307.
16. Lindsay DS, Zajac AM. Cryptosporidium infections in cats and dogs. Compend Contin Educ Pract Vet 2004;26:864-874.
17. McReynolds CA, Lappin MR, Ungar B, et al. Regional seroprevalence of Cryptosporidium parvum-specific IgG antibodies of cats in the United States. Vet Parasitol 1999;80:187-195.
18. Hill SL, Cheney JM, Taton-Allen GF, et al. Prevalence of enteric zoonotic organisms in cats. J Am Vet Med Assoc 2000;216:687-692.
19. Spain CV, Scarlett JM, Wade SE, et al. Prevalence of enteric zoonotic agents in cats less than 1 year old in central New York State. J Vet Intern Med 2001;15:33-38.
20. Scorza AV, Lappin MR. Detection of Cryptosporidium spp. in feces of cats and dogs in the United States by PCR assay and IFA (abst). J Vet Intern Med 2005;19:437.
21. Gookin JL, Stebbins ME, Hunt E, et al. Prevalence of and risk factors for feline Tritrichomonas foetus and Giardia infection. J Clin Microbiol 2004;42:2707-2710.
22. Thompson RCA. The zoonotic potential of Cryptosporidium. In: Thompson RCA, Armson A, Ryan UM, eds. Cryptosporidium: from molecules to disease. New York, NY: Elsevier, 2003;113-119.
23. Kirkpatrick CE, Laczac JP. Giardiasis in a cattery. J Am Vet Med Assoc 1985;187:161-162.
24. Gookin JL, Breitschwerdt EB, Levy MG, et al. Diarrhea associated with trichomonosis in cats. J Am Vet Med Assoc 1999;215:1450-1454.
25. Carey CM, Lee H, Trevors JT. Biology, persistence and detection of Cryptosporidium parvum and Cryptosporidium hominis oocysts. Water Res 2004;38:818-862.
26. Gookin JL, Nordone SK, Argenzio RA. Host responses to Cryptosporidium infection. J Vet Intern Med 2002;16:12-21.
27. Poonacha KB, Pippin C. Intestinal cryptosporidiosis in a cat. Vet Pathol 1982;19:708-710.
28. Lappin MR, Dowers K, Edsell D, et al. Cryptosporidiosis and inflammatory bowel disease. Feline Pract 1997;25:10-13.
29. Monticello TM, Levy MG, Bunch SE, et al. Cryptosporidiosis in a feline leukemia virus-positive cat. J Am Vet Med Assoc 1987;191:705-706.
30. Goodgame RW, Kimball K, Ou CN, et al. Intestinal function and injury in acquired immunodeficiency syndrome-related cryptosporidiosis. Gastroenterology 1995;108:1075-1082.
31. Burgess DE, et al. Cytotoxic and hemolytic effects of Tritrichomonas foetus on mammalian cells. Infect Immun 1990;58:3627-3632.
32. Yaeger MJ, Gookin JL. Histologic features associated with Tritrichomonas foetus-induced colitis in domestic cats. Vet Pathol 2005;42:797-804.
33. Asahi H, Koyama T, Arai H, et al. Biological nature of Cryptosporidium spp. isolated from a cat. Parasitol Res 1991;77:237-240.
34. Arai H, Fukuda Y, Hara T, et al. Prevalence of Cryptosporidium infection among domestic cats in the Tokyo metropolitan district. Jpn J Med Sci Biol 1990;43:7-14.
35. Goodwin MA, Barsanti JA. Intractable diarrhea associated with intestinal cryptosporidiosis in a domestic cat also infected with feline leukemia virus. J Am Anim Hosp Assoc 1990;26:335-337.
36. Lappin MR, Ungar B, Brown-Hahn B, et al. Enzyme-linked immunosorbent assay for the detection of Cryptosporidiumparvum IgG in the serum of cats. J Parasitol 1997;83:957-960.
37. Keith CL, Radecki SV, Lappin MR. Evaluation of fenbendazole for treatment of Giardia infection in cats concurrently infected with Cryptosporidium parvum. Am J Vet Res 2003;64:1027-1029.
38. Perez Trot G, Asanovic J, Petetta L. Resolucion de un caso complejo de diarrhea en una gata. Rev Med Vet 2002;84:73-74.
39. Brightman AH, Slonka GF. A review of five clinical cases of giardiasis in cats. J Am Anim Hosp Assoc 1976;12:492-497.
40. Nesvadba VJ. Giardiasis in a cat. Kleintier-Praxis 1979;24:177-179.
41. Brown RR, Elston TH, Evans L, et al. American Association of Feline Practitioners 2003 Report on Feline Zoonoses. Compend Contin Educ Pract Vet 2003;25:936-965.
42. Marks SL, Hanson TE, Melli AC. Comparison of direct immunofluorescence, modified acid-fast staining, and enzyme immunoassay techniques for detection of Cryptosporidium spp. in naturally exposed kittens. J Am Vet Med Assoc 2004;225:1549-1553.
43. Lappin MR, Jensen WA, Taton-Allen G. Comparison of Zn SO4 centrifugation, a fecal antigen assay, and an immunofluorescent antigen assay for diagnosis of giardiasis in cats (abst). J Vet Intern Med 2002;345.
44. Caccio SM, De Giacomo M, Pozio E. Sequence analysis of the beta-giardin gene and development of a polymerase chain reaction-restriction fragment length polymorphism assay to genotype Giardia duodenalis cysts from human faecal samples. Int J Parasitol 2002;32:1023-1030.
45. Leib MS, Zajac AM. Giardiasis in dogs and cats. Vet Med 1999;94:793-802.
46. Lappin MR, Calpin J. Laboratory diagnosis of protozoal diseases. In: Greene CE, ed. Infectious diseases of the dog and cat. Philadelphia, Pa: WB Saunders Co, 1998;437-441.
47. Zimmer JF, Burrington DB. Comparison of four techniques of fecal examination for detecting canine giardiasis. J Am Anim Hosp Assoc 1986;22:161-167.
48. Payne PA, Ridley RK, Dryden MW, et al. Efficacy of a combination febantel-praziquantel-pyrantel product, with or without vaccination with a commercial Giardia vaccine, for treatment of dogs with naturally occurring giardiasis. J Am Vet Med Assoc 2002;220:330-333.
49. Cirak VY, Bauer C. Comparison of conventional coproscopical methods and commercially coproantigen ELISA kits for the detection of Giardia and Cryptosporidium infections in dogs and cats. Berl Munch Tierarztl Wochenschr 2004;117:410-413.
50. Gookin JL, Foster DM, Poore MF, et al. Use of a commercially available culture system for diagnosis of Tritrichomonas foetus infection in cats. J Am Vet Med Assoc 2003;222:1376-1379.
51. Gookin JL, Birkenheuer AJ, Breitschwerdt EB, et al. Single-tube nested PCR for detection of Tritrichomonas foetus in feline feces. J Clin Microbiol 2002;40:4126-4130.
52. Farthing MJG. Clinical aspects of human cryptosporidiosis. Contrib Microbiol 2000;6:50-74.
53. Elitok B, Elitok OM, Pulat H. Efficacy of azithromycin dihydrate in treatment of cryptosporidiosis in naturally infected dairy calves. J Vet Intern Med 2005;19:590-593.
54. Hewitt RG, Yiannoutsos CT, Higgs ES, et al. Paromomycin: no more effective than placebo for treatment of cryptosporidiosis in patients with advance human immunodeficiency virus infection. Clin Infect Dis 2000;31:1084-1092.
55. Barr SC, Jamrosz GF, Hornbuckle WE, et al. Use of paromomycin for treatment of cryptosporidiosis in a cat. J Am Vet Med Assoc 1994;205:1742-1743.
56. Gookin JL, Riviere JE, Gilger BC, et al. Acute renal failure in four cats treated with paromomycin. J Am Vet Med Assoc 1999;215:1821-1823.
57. Diaz E, Mondragon J, Ramirez E, et al. Epidemiology and control of intestinal parasites with nitazoxanide in children in Mexico. Am J Trop Med Hyg 2003;68:384-385.
58. Ortiz JJ, Ayoub A, Gargala G, et al. Randomized clinical study of nitazoxanide compared to metronidazole in the treatment of symptomatic giardiasis in children from Northern Peru. Aliment Pharmacol Ther 2001;15:1409-1415.
59. Hawrelak J. Giardiasis: pathophysiology and management. Altern Med Rev 2003;8:129-142.
60. Shatto NL. Feline giardiasis (a case report). Vet Med Small Anim Clin 1981;76:1297-1298.
61. Zimmer JF. Treatment of feline giardiasis with metronidazole. Cornell Vet 1987;77:383-388.
62. Scorza AV, Lappin MR. Metronidazole for the treatment of feline giardiasis. J Feline Med Surg 2004;6:157-160.
63. Groman R. Metronidazole. Compend Contin Educ Pract Vet 2000;22:1104-1107.
64. Lindsay DS, Blagburn BL. Antiprotozoan drugs. In: Adams HR, Veterinary pharmacology and therapeutics. 8th ed. Ames: Iowa State University Press, 2001;993-994.
65. Caylor KB, Cassimatis MK. Metronidazole neurotoxicosis in two cats. J Am Anim Hosp Assoc 2001;37:258-262.
66. Harris JC, Plummer S, Lloyd D. Antigiardial drugs. Appl Microbiol Biotechnol 2001;57:614-619.
67. Schwartz RD, Donoghue AR, Baggs RB, et al. Evaluation of the safety of fenbendazole in cats. AmJ Vet Res 2000;61:330-332.
68. Plumb DC. Veterinary drug handbook. 5th ed. Ames, Iowa: Blackwell Publishing, 2005;1126-1128.
69. Stokol T, Randoph JF, Nachbar S, et al. Development of bone marrow toxicosis after albendazole administration in a dog and cat. J Am Vet Med Assoc 1997;210:1753-1756.
70. Scorza AV, Radecki SV, Lappin MR. Efficacy of a combination of febantel, pyrantel, and praziquantel for the treatment of kittens experimentally infected with Giardia species. J Feline Med Surg 2006;8:7-13.
71. Barr SC, Bowman DD, Frongillo MR, et al. Efficacy of a drug combination of praziquantel, pyrantel pamoate, and febantel against giardiasis in dogs. Am J VetRes 1998;59:1134-1136.
72. Olson ME, Hannigan C, Gaviller R, et al. The use of a Giardia vaccine as an immunotherapeutic agent in dogs. Can Vet J 2001;42:865-868.
73. Stein JE, Radecki SV, Lappin MR. Efficacy of Giardia vaccination in the treatment of giardiasis in cats. J Am Vet Med Assoc 2003;222:1548-1551.
74. Okhuysen PC, Chappell CL, Crabb JH, et al. Virulence of three distinct Cryptosporidium parvum isolates for healthy adults. J Infect Dis 1999;180:1275-1281.
75. Barr SC. Giardiasis. In: Greene CE, ed. Infectious diseases of the dog and cat. 3rd ed. New York, NY: Elsevier, 2005;736-742.
76. Anderson KA, Brooks AS, Morrison AL, et al. Impact of Giardia vaccination on asymptomatic Giardia infections in dogs at a research facility. Can Vet J 2004;45:924-930.
77. Glaser CA, Safrin S, Reingold A, et al. Association between Cryptosporidium infection and animal exposure in HIV-infected individuals. J Acquir Immune Defic Syndr Hum Retrovirol 1998;17:79-82.
78. The Centers for Disease Control and Prevention, Division of Parasitic Disease. Preventing cryptosporidiosis: a guide to water filters and bottled water. Available at: www.cdc.gov/ncidod/dpd/parasites/cryptosporidiosis/factsht_crypto_prevent_water.htm. Accessed Feb 21, 2006.