A challenging case: Endocarditis in a Boston terrier
Was this dog's tooth root abscess the cause of its severe, progressive systemic illness?
A 9-year-old 12-lb spayed female Boston terrier was presented to the emergency service at Angell Animal Medical Center for evaluation of lethargy and anorexia of one day's duration. The owners also reported progressive hindlimb ataxia.
The owners described the dog's hindlimbs as shaky for a few days, and by the afternoon prior to presentation, the dog could only take a few steps before collapsing. During the collapsing episodes, the dog vomited and urinated but did not appear to lose consciousness, according to the owners. The owners reported that there were no clinical signs of neurologic or cardiovascular disease two days before presentation.
HISTORY
Two weeks before presentation, the dog received its annual examination from its primary care veterinarian and was prescribed clindamycin (3.1 mg/kg orally twice daily) for treatment of a suspected tooth root abscess. The dog received the drug until vomiting began the day before presentation.
Long-term medical history included documentation of calculus and periodontal disease by the primary veterinarian, but a dental procedure was not pursued. A grade II heart murmur was also first diagnosed one year before; however, diagnostic tests, such as thoracic radiography or an echocardiogram, were not performed at that time to further evaluate and characterize the murmur.
INITIAL EXAMINATION FINDINGS
On presentation, the patient was quiet with slight mental dullness. The dog's temperature was minimally elevated at 102.8 F (39.3 C), and the dog was slightly tachycardic at 138 beats/min. Abnormal physical examination results included about 5% dehydration, pale mucous membranes, bilateral serous nasal discharge, a grade III/VI left apical systolic heart murmur, and harsh lung sounds bilaterally.
An oral examination revealed significant periodontal disease with severe dental calculi of the canine, premolar, and molar teeth as well as erythema of the gingiva characteristic of gingivitis. There was also gingival recession with root exposure of the maxillary fourth premolars and molars.
Neurologic examination results showed the patient to be mentally dull with a right-sided head tilt. The dog had pelvic limb ataxia, generalized paresis of the hindlimbs, and conscious proprioceptive deficits to the right pelvic limb. The patient would also sit with spinal pressure to the cervical and lumbar spine.
DIAGNOSTIC TESTS
Initial diagnostic tests included a complete blood count (CBC), serum chemistry profile, and venous blood gas analysis. The CBC revealed a normal white blood cell count; however, evaluation of a blood smear by a veterinary pathologist confirmed the presence of 1+ toxic change. Red blood cell morphology and platelets were adequate on the smear. The patient was also lymphopenic and anemic (Table 1). Abnormal serum chemistry profile values included elevated alkaline phosphatase activity, hypocalcemia, hyponatremia, hypochloremia, hypomagnesemia, and increased anion gap (Table 1). All the changes on the serum chemistry profile were considered mild. Despite a low total calcium concentration, the ionized calcium was within normal limits on the venous blood gas analysis. The only additional information from the venous blood gas analysis was mild respiratory alkalosis as indicated by the elevated pH and the low partial pressure of CO2 (Table 1).
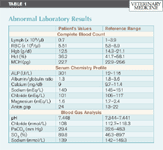
Table 1: Abnormal Laboratory Results
Thoracic radiography was attempted the evening of presentation but was aborted because of a collapsing episode. During the episode, the patient vomited and urinated, and its mucous membranes became pale.
INITIAL TREATMENT
The patient began receiving oxygen, and monitoring was begun with a continuous electrocardiogram (ECG) immediately after the collapsing episode. The patient was treated with pantoprazole (1 mg/kg intravenously once daily) and dolasetron (0.5 mg/kg intravenously once daily). Because of the concern of both neurologic disease and cardiac disease and because the patient did not appear clinically dehydrated despite vomiting episodes, fluid therapy was not initiated on presentation until further diagnostics could be performed.
Vitals, including mentation assessment, were performed every six hours with respiratory rate and effort being monitored every two hours. The patient had two more episodes of vomiting overnight. An intermittent tachyarrhythmia was noted on telemetry by a technician during one of the vomiting episodes, but the tachyarrhythmia had resolved by the time the veterinarian was notified and evaluated the ECG.
PROGRESSION OF NEUROLOGIC SIGNS
Ten hours after presentation, the patient was laterally recumbent and no longer able to ambulate without assistance. The dog could bear weight on all four limbs but would immediately lie down without support. Conscious proprioception was absent in both hindlimbs with decreased reflexes and forelimb rigidity. Evaluation by a neurologist confirmed an upper motor neuron spinal cord lesion with nonambulatory pelvic limb paresis. The lesion was localized to the T3-L3 spinal cord, although diffuse cerebrocortical disease was also suspected because of the dull mentation.
At this time, differential diagnoses included autoimmune or infectious myelitis and encephalitis, primary or metastatic neoplasia, or a vascular accident to the spinal cord and possibly brain.
ADDITIONAL DIAGNOSTIC TESTS
Thoracic radiography revealed mild left-sided cardiomegaly with a vertebral heart score of 12 (normal for dogs = 8.7 to 10.7) without evidence of congestive heart failure. The pulmonary parenchyma was unremarkable and mild hepatomegaly was appreciated on the included portion of the abdominal cavity.
At this time, the patient developed ventricular and supraventricular ectopy. Frequent isolates of ventricular premature complexes, isolated supraventricular premature complexes, and periods of accelerated idioventricular rhythm were noted on the ECG. The arrhythmias converted to sinus rhythm without intervention. Because of the combination of both neurologic and cardiovascular abnormalities, systemic disease was suspected.
DIAGNOSTIC RECOMMENDATIONS
An echocardiogram, abdominal ultrasonography, and magnetic resonance imaging with cerebrospinal fluid collection were recommended, but unfortunately, the patient's condition rapidly declined. Because of the severity of the patient's clinical signs, the rapid progression, and the cost associated with further diagnostic tests, the owners elected euthanasia and consented to an educational postmortem examination.
NECROPSY
At necropsy, thick tan-to-brown encrustations (dental calculus) adhered to the canine, premolar, and molar teeth. The maxillary fourth premolars and molars exhibited gingival recession up to 7 mm and were easily moveable with gentle digital pressure. Periodontal probing of the left maxillary first molar tooth indicated communication with the palatal root (measuring 8 mm in depth). The loss of normal periodontal structures to the level of the apex—thus, allowing communication of oral flora and the endodontic system of the tooth—is known as a class II perio-endo lesion.1 This tooth was easily extracted with digital pressure. The apex of the palatal root of this tooth was swabbed for culture.
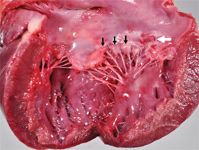
1. The left atrium of the heart contained a raised, roughened, plaque-like lesion (white arrow) at the base of the atrioventricular valve consistent with endocarditis and smooth nodular thickening (black arrows) along the free margins of the valve leaflets indicative of chronic valvular disease (endocardiosis).
Within the left atrium of the heart, a rough, slightly raised, red-and-tan, plaque-like lesion measuring about 1 cm in diamet you er was present on the endocardial surface of the left atrioventricular valve (Figure 1). The margins of the valve leaflets had smooth, nodular thickening consistent with endocardiosis. Multiple pinpoint-to-1-to-2-mm dark red foci were present on the epicardium and endocardium, within the renal cortex (Figure 2), and on the leptomeningeal surface of the cerebrum.
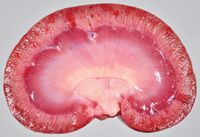
2. On the cut section of the kidney, multiple dark red pinpoint-to-1-to-2-mm foci were distributed throughout the renal cortex.
Histologic examination
The pulp cavity of the examined first molar contained granular necrotic debris, hemosiderin, nonstaining cholesterol clefts, and numerous gram-negative bacterial rods with lesser numbers of gram-positive cocci. The tooth roots were irregularly scalloped and heavily colonized by bacteria. The left atrioventricular valve had full-thickness infiltration and expansion by numerous neutrophils, fibrin, hemorrhage, and blood vessels lined by plump (reactive) endothelial cells (Figure 3). Gram-negative bacterial rods colonized the surface of the inflamed valve (Figure 3 inset), which had nonuniform damage to the endothelium. Discrete, unencapsulated foci of neutrophilic inflammation with associated hemorrhage, necrosis, and variable vascular fibrinoid necrosis were present in the myocardium, kidney (typically centered on renal glomeruli [Figure 4]), small intestine, leptomeninges, cerebrum, and spinal cord. Within the central nervous system, foci of suppurative inflammation were predominantly observed in grey matter. Gram-negative bacterial rods were rarely identified in affected glomeruli (Figure 4 inset) and were not observed in other affected tissues.
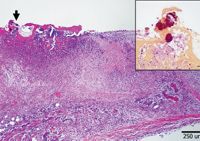
3. Histopathologic examination of the plaque on the atrioventricular valve revealed marked, full-thickness fibrinosuppurative inflammation with colonization by bacteria (arrow) (hematoxylin-eosin stain). Inset: Bacteria were gram-negative (Brown & Hopps stain; inset bar=25 microns).
Culture results
Aerobic culture of the suspected tooth root abscess yielded abundant growth of normal respiratory flora as well as Pasteurella species, Acinetobacter lwoffii, and Enterobacter cloacae. Aerobic culture of the right kidney and heart also grew Pasteurella pneumotropica and Enterobacter cloacae. The right kidney and heart also grew Klebsiella pneumoniae, Cronobacter sakazakii, a gram-positive cocci that was not further characterized, and Clostridium perfringens.
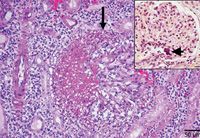
4. Histopathologic examination of the kidney revealed intense fibrinosuppurative inflammation centered on glomeruli (arrow) and extending into the surrounding renal interstitium (hematoxylin-eosin stain). Inset: Rare gram-negative bacterial colonies were identified in renal glomeruli (arrowhead) (Brown & Hopps stain; inset bar=25 microns).
DIAGNOSIS
Given the similar morphology and gram staining of bacterial organisms identified in the pulp canal and heart valve in histologic sections as well as the bacterial culture of two common bacteria from the apex of the palatal root of the left maxillary first molar tooth and the pooled kidney and heart tissues, it is postulated that bacteremia secondary to periodontal disease was the cause of endocarditis in this case. Endocarditis subsequently led to septic embolization with multiorgan involvement.
DISCUSSION
Periodontal disease
Periodontal disease is one of the most common health problems in pets and has been reported to affect more than 75% of dogs.2 A recent multihospital report, in which the facilities primarily see healthy pets, found that dental calculus was the top diagnosis affecting 78% of dogs older than 3 years.3 Inoffensive breath and white teeth are important to pet owners, but the numerous health benefits resulting from a thorough evaluation and appropriate periodontal disease treatment are often overlooked. Periodontal disease not only affects the health of the oral cavity but may also be associated with serious systemic illness.
Many studies in human literature have looked at the potential of periodontal disease to cause systemic illness. Periodontal disease has been associated with infective endocarditis, aspiration pneumonia, necrotizing fasciitis, diabetes, and atherosclerosis.4 But the potential association between periodontal disease and systemic illness has not been as well-researched in veterinary medicine. Postmortem evaluation of 45 dogs did show significance between the extent of periodontal disease and histopathologic changes in other organs, including the kidneys, myocardium, and liver.5 Another study found that increasing severity of periodontal disease in dogs was associated with increased C-reactive protein concentrations, a systemic inflammatory marker, and renal indices. The study also found a decrease in the C-reactive protein concentrations after appropriate treatment of periodontal disease, showing that there may be a systemic impact with periodontal disease.6
Endocarditis
Compared with periodontal disease, endocarditis is rare in dogs, with prevalence ranging from 0.05% to 6.6%.7 The pathogenesis of bacterial endocarditis in dogs is complex, and our understanding of it is mostly extrapolated from human data. Many people with endocarditis have predisposing cardiac conditions. Since normal vascular endothelium is resistant to bacterial adhesion and colonization, underlying heart defects may cause damage to vascular endothelium secondary to turbulent or high-velocity blood flow.8
In dogs, as in people, damage to the vascular endothelium is an important step in bacterial colonization. Thus, it is interesting to note that, unlike in people, most underlying heart diseases do not appear to predispose dogs to infectious endocarditis.9 In this patient, the previously reported heart murmur was likely due to endocardiosis of the mitral valve. This assumption is made based on the patient's signalment and history and was confirmed by postmortem examination findings. The prevalence of endocarditis in dogs with endocardiosis is low.9 However, the prevalence of bacterial endocarditis is low in general. As endocardiosis progresses, the endothelium undergoes uneven damage, leading to areas of denuded valve and exposure of the basement membrane or collagen matrix.10,11 Endocardiosis and subsequent valvular endothelial damage could theoretically allow bacterial adhesion and colonization. Thus, the role of underlying endocardiosis in the development of bacterial endocarditis in this patient is uncertain.
A diagnosis of bacterial endocarditis can be difficult to make in antemortem patients. Endocarditis is definitively diagnosed based on pathology of the heart valve or when the patient meets two of the major criteria or one major and two minor criteria (Table 2).12 In one study, only 44% of dogs with endocarditis had temperatures > 103 F (39.4 C).13 A new or previously undiagnosed heart murmur often raises the suspicion of endocarditis; however, 59% of dogs with bacterial endocarditis do not have a new murmur on physical examination.14 Another study found that only 33% of cases of bacterial endocarditis had a new heart murmur or change in intensity of an existing heart murmur.13 CBC results that support a diagnosis of endocarditis include monocytosis, anemia, and thrombocytopenia, which are nonspecific findings.7 Echocardiography is the single most useful diagnostic test for endocarditis and is reported to have sensitivities as high as 90%.9,12 However, an echocardiogram alone is usually insufficient in diagnosing infective endocarditis in most cases.12

Table 2: Criteria for Diagnosing Endocarditis*
In this case, the patient did not have a temperature > 103 F (39.4 C) on presentation. This finding may have been influenced by the antibiotic treatment the patient was receiving for two weeks before presentation. The patient also did not have a new heart murmur, as its record indicated the presence of a heart murmur for at least a year. Although monocytosis is present in more than 90% of cases,7 neither monocytosis nor thrombocytopenia were present in this case. Thus, the only findings that supported the diagnosis of endocarditis in this patient were anemia and an increase in the intensity of the heart murmur, although the latter may reflect a difference in the practitioners evaluating the murmur or in the patient's systemic condition.
Patients with bacterial endocarditis frequently have many nonspecific clinical signs on presentation, which is true in this case as well. Many of these clinical signs are due to septic embolization to a variety of organ systems. It has been reported that 32% of dogs with endocarditis had septic emboli found on necropsy.13 The kidneys are the most common organ to suffer ischemic insult because of septic emboli. Immune-complex glomerulonephritis is also commonly found on necropsy.7 Neurologic signs also occur because of septic emboli. In the case reported here, bacterial colonies were still present in the kidney despite antibiotic therapy. Meningoencephalitis was also present, but no bacterial emboli were detected.
The patient's collapsing episode may be explained by cardiac arrhythmia. The degree of disease present in the myocardium, including neutrophilic inflammation and necrosis, may have led to significant arrhythmias and subsequent collapse. Neurally mediated syncope, also called vasovagal syncope, may have also occurred. Increased vagal tone—in this case vomiting—is associated with acute decreases in systemic blood pressure and heart rate. Because of the patient's severe neurologic signs, including dull mentation, other causes of collapse such as central nervous system bacterial thromboemboli cannot be ruled out.
The relationship between periodontal disease and endocarditis
Although a causal relationship between periodontal and cardiovascular disease has been difficult to establish in veterinary medicine, numerous human studies have found an association between these conditions.15,16 About 80% of all studies found a significant positive association between periodontal disease and cardiovascular disease in people.15 Inflammation and endothelial dysfunction are believed to play a major role in periodontal disease leading to cardiovascular disease, and one study did find a causal relationship between periodontal disease and endothelial dysfunction.15 Even the reduction of pathogen burden with antibiotic therapy once endothelial dysfunction has occurred does not seem to lower the risk of cardiovascular morbidity and mortality.15 However, the treatment of severe periodontitis is shown to reverse endothelial dysfunction.17 Thus, the recommendation in human medicine for minimizing the risk of periodontal disease leading to cardiovascular disease is to prevent endothelial dysfunction from occurring by optimizing oral health.
There is some evidence that canine periodontal disease can affect the cardiovascular system. Common bacterial species of the oral cavity, such as Streptococcus species and Staphylococcus species, have been isolated from the heart valves of dogs.13 One study found that the risk of endocarditis was about six times higher in dogs with severe periodontal disease than in those without evidence of periodontal disease or with mild periodontal disease. However, there were several limitations to this study, including an inability to standardize the accuracy of diagnosis of cardiovascular-related events.18
The most recent guidelines from the American Heart Association for the prevention of infective endocarditis have attributed the association of dental disease and endocarditis to randomly occurring bacteremia during routine daily activities, rather than from bacteremia caused by invasive oral procedures, including dental scaling and polishing.19 It is postulated that maintaining optimal oral health and hygiene will reduce the prevalence of bacteremia.19 The American Veterinary Dental Society recommends routine home care including brushing, professional oral health assessment, and appropriate periodontal treatment in our veterinary patients as well in order to reduce the bacterial load.20
We should not only inform owners of the many potential benefits of maintaining a pet's oral health but should also emphasize the potential for periodontal and endodontic disease to result in serious local and systemic disease. Client education is an important key to improving overall compliance in what is often viewed as an elective procedure.
CONCLUSION
Periodontal disease in veterinary patients is generally not considered life threatening. However, the case reported here demonstrates how severe periodontitis can affect the overall health of a patient, ultimately leading to significant morbidity and death. In this case, severe periodontal disease was the suspected source of bacteremia leading to endocarditis.
Although bacterial endocarditis does not have a high prevalence in veterinary patients, it is associated with a poor prognosis. Maintaining good oral health is an important proactive measure by which pet owners and veterinarians can attempt to reduce the impact that advanced periodontal disease may have on the systemic health of our patients.
Michelle Fulks, DVM
Cape Cod Veterinary Specialists
11 Bourne Bridge Approach
Buzzards Bay, MA 02532
Rebecca Quinn, DVM, DACVIM
Pamela J. Mouser, DVM, MS, DACVP
Curtis A. Stiles, DVM, DAVDC
Angell Animal Medical Center
350 South Huntington Ave.
Boston, MA 02130
REFERENCES
1. Wiggs R, Lobprise H. Veterinary dentistry: principles and practice. New York, N.Y.: Wiley-Blackwell, 1997;287.
2. Colmery B III, Frost P. Periodontal disease. Etiology and pathogenesis. Vet Clin North Am Small Anim Pract 1986;16:817-834.
3. Banfield Pet Hospital. State of Pet Health 2011 Report, Volume 1.
4. Slots J. Update on general health risk of periodontal disease. Int Dent J 2003;53(Suppl 3):200-207.
5. DeBowes LJ, Mosier D, Logan E, et al. Association of periodontal disease and histologic lesions in multiple organs from 45 dogs. J Vet Dent 1996;13:57-60.
6. Rawlinson JE, Goldstein RE, Reiter AM, et al. Association of periodontal disease with systemic health indices in dogs and the systemic response to treatment of periodontal disease. J Am Vet Med Assoc 2011;238:601-609.
7. Peddle G, Sleeper MM. Canine bacterial endocarditis: a review. J Am Anim Hosp Assoc 2007;43:258-263.
8. Karchmer AW. Infective endocarditis. In: Braunwald E, ed. Heart disease: a textbook of cardiovascular medicine. 7th ed. Philadelphia, Pa: W.B. Saunders Co, 2005;1077-1099.
9. Kittleson MD. Infective endocarditis. In: Kittleson MD, Kienle RD, eds. Small animal cardiovascular medicine. St. Louis, Mo: C.V. Mosby, 1998;402-412.
10. Kittleson, MD. Myxomatous atrioventricular valvular degeneration. In: Kittleson MD, Kienle RD, eds. Small animal cardiovascular medicine. St. Louis, Mo: C.V. Mosby, 1998;297-317.
11. Corcoran BM, Black A, Anderson H, et al. Identification of surface morphologic changes in the mitral valve leaflets and chordae tendineae of dogs with myxomatous degeneration. Am J Vet Res 2004;65:198-206.
12. Macdonald KA. Infective endocarditis. In: Bonagura JD, Twedt DC, eds. Kirk's current veterinary therapy XIV. Philadelphia, Pa: W.B. Saunders Co, 2009;790.
13. Peddle GD, Drobatz KJ, Harvey CE, et al. Association of periodontal disease, oral procedures, and other clinical findings with bacterial endocarditis in dogs. J Am Vet Med Assoc 2009;234:100-107.
14. Calvert CA. Valvular bacterial endocarditis in the dog. J Am Vet Med Assoc 1982;180:1080-1084.
15. Offenbacher S, Beck JD. A perspective on the potential cardioprotective benefits of periodontal therapy. Am Heart J 2005;149:950-954.
16. Beck JD, Offenbacher S. Systemic effects of periodontitis: epidemiology of periodontal disease and cardiovascular disease. J Periodontol 2005;76(Suppl 11):2089-2100.
17. Seinost G, Wimmer G, Skerget M, et al. Periodontal treatment improves endothelial dysfunction in patients with severe periodontitis. Am Heart J 2005;149:1050-1054.
18. Glickman LT, Glickman NW, Moore GE, et al. Evaluation of the risk of endocarditis and other cardiovascular events on the basis of severity of periodontal disease in dogs. J Am Vet Med Assoc 2009;234:486-494.
19. Wilson W, Taubert KA, Gewitz M, et al. Prevention of infective endocarditis: guidelines from the American Heart Association: a guideline from the American Heart Association Rheumatic Fever, Endocarditis, and Kawasaki Disease Committee, Council on Cardiovascular Disease in the Young, and the Council on Clinical Cardiology, Council on Cardiovascular Surgery and Anesthesia, and the Quality of Care and Outcomes Research Interdisciplinary Working Group. Circulation 2007;116:1736-1754. Available at: http://circ.ahajournals.org/content/116/15/1736.full.
20. American Veterinary Dental Society. Periodontal disease. Available at: www.avds-online.org/info/periodontaldisease.html. Accessed Nov 12, 2011.