Clinical pearls for managing anticoagulant rodenticide intoxication (Proceedings)
The anticoagulant class of rodenticides comprises 95% of the rodenticides used in the United States.
The anticoagulant class of rodenticides comprises 95% of the rodenticides used in the United States. The remaining classes of rodenticides work by many disparate mechanisms. The most common of these other classes of rodenticides include:
- Bromethalin: This chemical is in its own class. It poisons the central nervous system by blocking the ability of neurons to recycle ATP. This leads to cerebral edema, paralysis and seizures. There is no antidote.
- Hypercalcemics: These chemicals are forms of vitamin D (most common is cholecalciferol). Vitamin D increases the absorption of calcium from the intestinal tract, leading to hypercalcemia. Hypercalcemia leads to mineralization of various tissues throughout the body. Renal failure ensues.
- Phosphides: In the acidic environment of the stomach, phosphides (most commonly zinc phosphide) are broken down to release toxic phosphine gas. This gas has corrosive effects to the gastrointestinal tract, is a direct cytotoxin and leads to methemoglobinemia.
It is important to note, that the appearance of rodenticide bait cannot differentiate between different classes of rodenticide. For example, anticoagulant and bromethalin baits can both be green.
Of the anticoagulant class of rodenticides, these toxins can be further broken down into subclasses based on their chemical composition and half-lives.
- First generation hydroxycoumarins: The original anticoagulant rodenticides were derivatives of coumarin. They have short half-lives and require either a single high dose or consecutive days of intake to cause toxicity. Examples include warfarin and coumatetralyl.
- Second generation hydroxycoumarins: The second generation was developed for killing Norway rats that had become resistant to warfarin. They have long half-lives and require lower doses to cause toxicity. Examples include brodifacoum and bromadiolone.
- Indandiones: This chemical family is similar in half-life as the second generation hydroxycoumarins. Examples include chlorophacinone and diphacinone.
A major concern about rodenticides, regardless of their mechanism of action, is their ability to potentially cause relay toxicity. Relay or secondary toxicity occurs when a predator eats poisoned prey. Dogs and housecats are at lower risk, as rats and mice do not make a large percentage component of their diet. However, the situation is more serious for wildlife and barn cats. These predators are at risk for developing relay toxicity. Second generation hydroxycoumarins are the most likely of the anticoagulant rodenticides to cause relay toxicity because of their relatively long half-lives and lower toxic doses.
Because of concerns over relay toxicity as well as concern for human children who encounter and ingest rodenticides, in 2008 the Environmental Protection Agency released new guidelines for rodenticides. The Risk Mitigation Decision for Ten Rodenticides tightened the regulation of household rodenticides. Baits could only be sold to the public as blocks and must be sold with a station. The public could not purchase bait blocks larger than 1 lb. in size. Finally, second generation hydroxycoumarins could not be sold to the public. (These restrictions did not apply to professional exterminator products.) Products that did not meet the new guidelines by 2011 were banned. As a result of this law, there unfortunately has been an increase in the amount of bromethalin intoxications reported to animal poison control centers.
All anticoagulant rodenticides inhibit the enzyme vitamin K1 epoxide reductase. Vitamin K1 epoxide reductase is an enzyme involved in recycling oxidized vitamin K1 (epoxide) back to its reduced form (hydroquinone). Hydroquinone is the cofactor in the carboxylation of coagulation factors II, VII, IX, and X. When vitamin K1 recycling is interrupted, the rate of secretion of epoxide exceeds the rate of dietary intake and enteric production and vitamin K1 deficiency results.
Figure 1: Vitamin K1 recycling
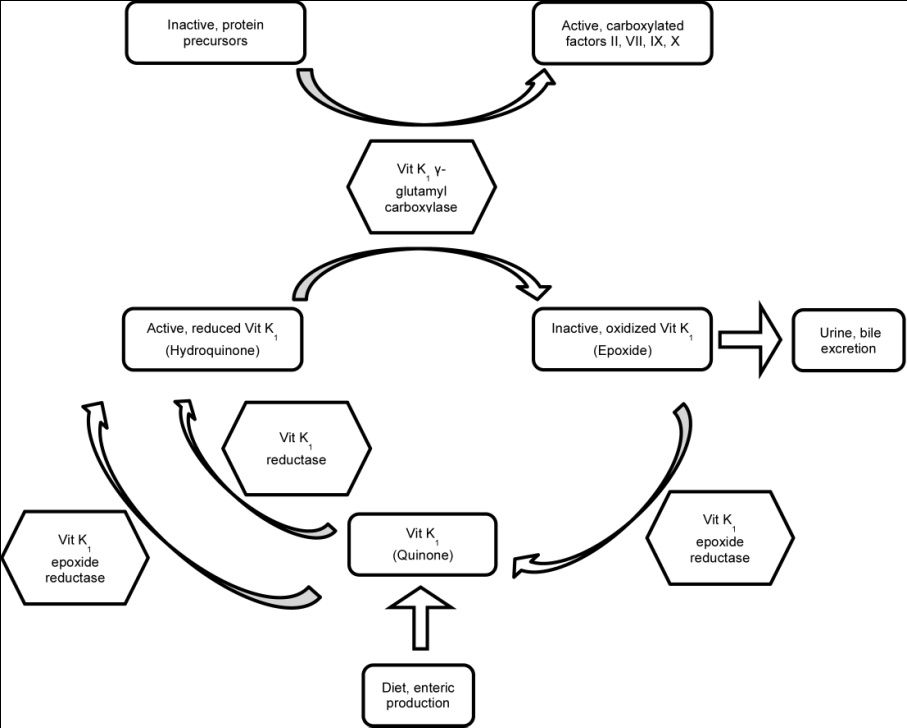
The rate at which activated vitamin K1-dependent coagulation factors become deficient depends on their half-life. Factor VII has the shortest half-life at 6.2 hours whereas factor II has the longest half-life at 41 hours. Clinical signs of anticoagulant rodenticide will typically start to manifest starting 72 hours following ingestion. Clinical signs will vary depending on the location of hemorrhage. Presenting complaints are more commonly vague (e.g. lethargy, anorexia, weakness) rather than dramatic (e.g. hemoptysis, hematemesis, epistaxis). Anticoagulant rodenticides, since they inhibit the secondary hemostasis, will tend to cause large hematomas and cavitary hemorrhage (e.g. peritoneal space, pleural space, pericardium, joints, or eye). Excessive bleeding will be noted following venipuncture. Death occurs due to hemorrhage shock, cardiac tamponade, respiratory embarrassment or central nervous system dysfunction.
When collecting a history of a patient who was seen to recently ingest a rodenticide, ask the owner to present the original packaging of the rodenticide, if possible. This will allow for proper identification and treatment of the intoxication (e.g. anticoagulant vs hypercalcemic rodenticide). Most packaging will also have a toll free number to call for information about the product and treatment recommendations.
When collecting a history of a patient displaying a coagulopathy, it is very important to not ask leading questions. For example, “Is there rat poison anywhere on your property?” is a superior question compared to “Is there any chance your pet got into rat poison?” If you ask the latter question, many owners will think their pet is “too smart” or “too well-behaved” to have ingested rodenticide that they put out. In fact, one should always have anticoagulant rodenticide as a differential in any patient presenting with a coagulopathy regardless of the history obtained from the owner.
Diagnostic tests commonly performed in the patient presenting for hemorrhage include a complete blood count to assess the degree of anemia and the platelet count, a serum biochemistry to assess for evidence of liver failure, coagulation testing to assess for coagulation factor deficiencies, and radiography and/or ultrasound to identify the extent of hemorrhage. When drawing blood from a hemorrhaging patient, use a peripheral vein only. A compressive bandage should be applied over the venipuncture site. In addition to being unable to apply a compressive bandage over the jugular vein, the other reason this vein should not be used is that a hematoma large enough to compress the trachea and cause dyspnea can result.
There are three coagulation tests commonly available for veterinary patients: the ACT, PT, and APTT. The PT will become elevated first, due to the short half-life of factor VII. The PT needs factors to be decreased to less than 30% of normal levels, which takes 36 hours. The ACT is the least sensitive test as it requires the coagulation factors to be decreased to less than 10% of normal levels, which takes 72 hours. Specific factor analysis on occasion may be performed if a hereditary coagulopathy (e.g. hemophilia) is suspected. There are also spectrophotometric tests that can be performed to identify anticoagulant rodenticides in the blood, however these test are rarely used for confirmation. A combination of ruling out other causes of coagulopathy and response to treatment is the method by which most cases are presumptively diagnosed.
If anticoagulant rodenticide ingestion is witnessed by the owner, performing decontamination is recommended whether or not it is known if a toxic dose was ingested. While the toxic dose of many anticoagulant rodenticides is known, it is often challenging to determine how much the patient actually ingested. Emesis can be induced if ingestion occurred within the past 6 hours. In dogs, apomorphine is the recommended emetic. Syrup of ipecac is not recommended as vomiting can be prolonged. Hydrogen peroxide is also no longer recommended because it can cause severe esophagitis and gastritis. In cats, xylazine is the recommended emetic. To encourage emesis, the abdomen can be palpated deeply. In addition, briskly walking a dog can be helpful.
After emesis has subsided, activated charcoal should be given to bind any rodenticide remaining in the gastrointestinal tract. Using an activated charcoal product that contains 70% sorbitol will act as a cathartic to speed transit through the gastrointestinal tract. Some dogs will willingly eat activated charcoal from a bowl. If you mix canned food or ice cream in with the charcoal, you will increase its palatability, but decrease its absorptive capacity. Therefore, this is not recommended. For patients that will not willingly eat the charcoal the options are to syringe feed the charcoal or place an orogastric tube for charcoal administration. It is recommended to anesthetize the patient if performing orogastric delivery of the charcoal to directly visualize that the tube is in the esophagus rather than the trachea. Instillation of charcoal into the trachea is can be fatal.
After decontamination, there are two schools of thought as to how to further treat a patient with acute rodenticide ingestion. One option is to check PT 48 hours following ingestion. If a toxic dose was ingested, the PT will be prolonged, but hemorrhage will not have started yet. If the PT is prolonged, vitamin K1 therapy should be started. If the PT is normal, no further treatment is needed. The other option is to immediately begin prophylactic vitamin K1 therapy. The length of treatment varies based on the type of anticoagulant rodenticide ingested. If the type was unknown, then treatment should continue for a minimum of 4 weeks. The PT should be checked 48 hours after cessation of vitamin K1 therapy. If the PT is prolonged, treatment should be continued for another 2 weeks before retesting. With the first option, unnecessary treatment is avoided and is less expensive. The second option is safest for the patient, as it is not dependent on owner compliance to appear for their recheck examination.
For a patient with a coagulopathy from anticoagulant rodenticide ingestion, decontamination will not be beneficial, as the rodenticide will have long since left the gastrointestinal tract. The coagulopathy will need to be corrected with transfusion of fresh frozen plasma or fresh whole blood. Administering vitamin K1 alone will not replenish the deficient coagulation factors rapidly enough to stop hemorrhaging. Vitamin K1 therapy should be instituted alongside transfusion therapy, however. If giving injectable vitamin K1 it should only be administered subcutaneously, never intravenously. Intravenous administration can trigger an anaphylactic reaction. Divide the vitamin K1 injections into multiple sites and use a small gauge needle. This will diminish the pain associated with administration and risk of hemorrhage. Oral vitamin K1 should be given with a fatty meal to increase its absorption.
The last cornerstone of treating the coagulopathic patient is supportive care. Dyspnic patients may require supplemental oxygen. If possible, centesis to remove pleural or peritoneal hemorrhage is not performed. Centesis may cause further hemorrhage and the patient will not be able to reabsorb the blood to autotransfuse themselves. Patients should be strictly cage rested until their coagulation times have normalized so as to limit any trauma-induced hemorrhage and to aid in monitoring.
When treated aggressively and appropriately, anticoagulant rodenticide ingestion carries an excellent prognosis. 80-90% of patients survive, even when presenting coagulopathic. However, death may rapidly occur if hemorrhage occurs into a vital area (e.g. the brain) before the coagulopathy is corrected. The importance of continuing vitamin K1 therapy for the proscribed length of treatment should be emphasized to owners at discharge. They may be tempted to discontinue it as their pet will appear normal to them if they happen to miss a dose.