Current treatment options for dogs with appendicular osteosarcoma
Although amputation has traditionally been used to palliatively manage affected dogs, treatment modalities including limb salvage, radiation therapy, and chemotherapy are evolving. This overview of emerging therapies will help you educate owners about treatment options for dogs with appendicular osteosarcoma.
Osteosarcoma is estimated to occur in more than 8,000 dogs a year in the United States.1 Although amputation has traditionally been used to palliatively manage affected dogs, treatment modalities including limb salvage, radiation therapy, and chemotherapy are evolving. This overview of emerging therapies will help you educate owners about treatment options for dogs with appendicular osteosarcoma.
CANINE OSTEOSARCOMA: AN OVERVIEW
Osteosarcoma is the most common primary bone neoplasia affecting dogs and accounts for nearly 6% of all canine malignancies.2 Large and giant breeds are more commonly affected, and males are more often affected than females by a ratio of 1.5:1.3-6 Although osteosarcoma has been reported in dogs as young as 6 months of age,7 there is a bimodal age distribution with the initial peak at 3 years and the second peak at 8 years.8 If left untreated, most dogs will be euthanized within three months of diagnosis.5,8 Osteosarcomas are classified as simple (bone formed in collagenous matrix), compound (both bone and cartilage are present), or pleomorphic (anaplastic cells predominate, with only small islands of osteoid present).9 Classification has also been based on cell type and activity (osteoblastic, chondroblastic, or fibroblastic), radiographic appearance (lytic, sclerotic, or mixed), or origin (central, juxtacortical, or periosteal).9
Clinically, osteosarcoma is characterized by aggressive local bone destruction with invasion into the surrounding soft tissues.9 Seventy-five percent of tumors arise in the metaphyseal region of the long bones.5,8,9 The remaining 25% occur in the axial skeleton or soft tissues.5,8,10 The distal radius is the most common site in dogs.5,8,9 Other less common appendicular sites include the proximal humerus, distal ulna, proximal or distal femur, and proximal or distal tibia. Osteosarcomas are typically rapidly growing, painful tumors.9
Grossly, central or interosseous osteosarcomas have a gray-white appearance and contain variable amounts of mineralized bone.9 Rapidly growing intramedullary osteosarcomas often have large pale areas of infarction and irregular areas of hemorrhage.9 Neoplastic tissue tends to fill the medullary cavity of the metaphysis and can extend proximally and distally. Typically, however, this extension does not penetrate growth plates or articular cartilage to enter the joint space.9 Cortical bone is destroyed with varying amounts of new reactive bone surrounding the area, while neoplastic cells penetrate and undermine the periosteum.9 New bone formation may be abundant and widespread or minimal.9
Radiographic evidence of metastasis is often not present at the time of initial diagnosis.1 Common sites of metastasis are the lungs and other skeletal tissues. Metastasis has also been reported in the visceral organs, brain, and subcutaneous tissues.1 Although less than 10% of affected dogs have radiographically detectable metastasis at the time of presentation, 90% of dogs die of metastatic disease within one year of diagnosis if not treated with chemotherapy and surgery.5,8 This suggests that micrometastatic disease is usually present in the lungs at the time of diagnosis, possibly in a dormant state.5,8 Depending on the location and stage of the disease, surgery, radiation therapy, and chemotherapy can be used in a variety of treatment protocols.
AMPUTATION
Amputation is the most common surgical treatment for appendicular osteosarcoma and is recommended for most dogs with osteosarcoma. Limb amputation provides pain relief, relatively short anesthesia times, a low risk of surgical and postoperative complications, a short convalescence, lower costs than limb-sparing surgery, and a limited risk of tissue contamination or local recurrence because of incomplete resection.11 Some owners, however, are reluctant to pursue amputation.12,13 This reluctance is unfortunate since most dogs, including the larger breeds, function well after amputation, and owners who elect amputation are typically satisfied with their pets' quality of life after the procedure.11,14 Body size, age, and forelimb vs. hindlimb do not appear to influence the rate of recovery, owner satisfaction, or the ability of a dog to adapt after amputation.12 Poor candidates for amputation include dogs with advanced pulmonary metastasis, marked or severe orthopedic or neurologic problems in other limbs, and severe obesity.15
LIMB-SPARING PROCEDURES
Limb-sparing, or limb-salvage, procedures for cancer patients are performed in an attempt to resect the primary lesion and preserve a functional, pain-free limb.16 These procedures are often considered when adverse circumstances preclude or owners refuse amputation. Limb-sparing procedures described to treat appendicular osteosarcoma in dogs include frozen cortical bone allograft implantation,17 pasteurized tumoral autografting,18,19 bone transport osteogenesis,20,21 and endoprosthesis.22 Most limb-sparing procedures performed to treat osteosarcoma in dogs involve lesions affecting the distal radius.16 Although local tumor recurrence after resection ranges from 20% to 40% in dogs undergoing limb-sparing procedures,14,16,17 this recurrence does not appear to decrease survival rates.11,17 Previous studies have shown that with adjunctive chemotherapy, no significant difference exists in survival rates between dogs undergoing amputation and dogs undergoing limb-sparing procedures.11,17 Methods to prevent local recurrence include systemic, regional, or local presurgical and postsurgical chemotherapy and preoperative radiation therapy.1
Proper biopsy techniques should be followed when you are considering limb-sparing procedures. If owners express interest in limb-sparing, consider sending the patient to a referral institution for the biopsy procedure. Ideally, the biopsy should be performed by the same surgeon who will ultimately perform the limb-sparing surgery. A searchable directory of diplomates of the American College of Veterinary Surgeons can be found at www.acvs.org. Techniques include open incisional biopsy, closed (hypodermic needle or Jamshidi biopsy needle) biopsy, and Michelle trephine biopsy.23-25 We prefer the closed technique with a Jamshidi biopsy needle. This technique can help minimize the risk of complications such as hematoma formation, infection, and pathologic fractures.25 Site selection should be based on evaluation of radiographs and consideration of subsequent treatments. Biopsy site selection is critical for lesions of the distal radius. Failure to place the biopsy tract in the correct location can compromise a successful limb-sparing procedure. Biopsies of distal radial lesions should be performed at the craniolateral aspect of the distal antebrachium and should not include a biopsy of the distal ulna unless the primary tumor is located within the ulna.
The clinician should review the radiographs before the biopsy and have the radiographs available for reference during the procedure. The center of the radiographic lesion should be targeted as the site most likely to yield a diagnosis.25 Areas of dense reactive bone should be avoided.25 The skin incision should be as small as possible and positioned in a location where the biopsy tract and any potentially seeded tumor cells can be easily resected en bloc with the tumor at the time of surgery.25
Proper case selection for limb-sparing surgery is essential for a favorable outcome. Several criteria are used to determine case eligibility. Tumors that radiographically involve greater than 50% of the bone's length are not amenable to adequate resection because of insufficient tissue for reconstruction.26 Extensive invasion into adjacent soft tissues, especially the palmar nerves, vessels, and tendons, will not allow adequate resection with concurrent preservation of essential neurovascular bundles to the paw.11,26 Unstable or catastrophic pathologic fractures result in local tumor dissemination and seeding of tumor cells, making complete excision of tumor-contaminated tissue difficult.27 Small pathologic fractures or telescoping collapse of lytic bone is commonly observed on preoperative radiographs; these minor pathologic fractures do not preclude limb-sparing surgery. Local infection and radiographic evidence of metastasis are also circumstances under which limb-sparing surgery is not recommended.7,11,26 When in doubt, it is best to seek the opinion of a specialist who regularly performs limb-sparing surgery to ensure appropriate case eligibility.
Cortical bone allograft implantation
The most common limb-sparing procedure used to treat distal radial osteosarcoma is cortical bone allograft implantation.16,17 In this procedure, a fresh-frozen (not preserved) cortical bone segment is cut to match the length of the excised tumor segment and affixed to the host bone with a bone plate and screws (Figure 1).17,26 Cortical bone allografts have been used in dogs with osteosarcoma affecting the distal radius, proximal humerus, ulna, and scapula; however, only those in the distal radius location have been widely successful.16
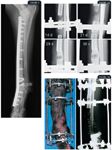
FIGURE 1. A lateral radiograph of a dog distal forelimb after cortical bone allograft implantation for the treatment of a distal radial osteosarcoma. The proximal five cortical bone screws fasten the bone plate to the remaining radius. The six central bone screws are placed into the allograft. The five distal bone screws fasten the plate to the radial carpal bone and the third metacarpal bone. FIGURE 2A. Serial radiographs of a dog with a distal radial osteosarcoma undergoing bone transport osteogenesis to replace a subtotal radial defect with regenerate bone. The radiographs were obtained 14, 21, 28, and 35 days after surgery. Note the movement of the transport segment distally and the subsequent regenerate bone formation. The radius, ulna, and transport segment are secured within a circular external skeletal fixation frame. FIGURE 2B. A postoperative photograph and a corresponding radiograph of a forelimb undergoing bone transport osteogenesis.
Dogs usually attempt to bear weight on the affected limb within 24 hours of surgery and gradually regain normal limb function within one or two months after surgery.11,16,17,26 Nontumor-related complications associated with cortical bone allograft limb-sparing procedures include infection, fracture of the host bone, rejection of the allograft, nonunion, or implant loosening or failure.16,28 Infection is the most common of these complications.16,28 About half the dogs undergoing cortical bone allograft implantation develop an infection at some point after surgery.16 Factors contributing to the high rate of infection include extensive surgical resection, use of a nonviable cortical bone allograft and large metallic implants, infusion of the allograft marrow cavity with bone cement to improve screw purchase, paucity of adjacent soft tissues to supply sufficient vasculature in the distal extremity, self-trauma, and the administration of adjunctive chemotherapy.16,28,29 Complete resolution of infection is extremely difficult because of bacteria residing in the allograft and in the implant-associated biofilm.16
Although 75% of infections can be treated with basic wound management, bandaging, and antibiotic therapy, the remaining 25% require additional surgery. Resolving the clinical signs related to the infection is sometimes achieved by removing the allograft or by implanting antibiotic-impregnated polymethyl methacrylate beads.30 For dogs that do not respond to these approaches, limb amputation may be the only option.16 It has been noted that dogs that develop infection at the surgical site have significantly longer survival times and higher local control rates than dogs that do not develop infections.16,31,32 The reason for this difference in survival and local tumor control is thought to have an immunologic basis.16 Although the effects of infection and the immune system are an area for future study, postoperative infection is not desirable because the quality of life for dogs with infected allografts is generally compromised by periods of pain, lameness, and drainage and the necessity for continual bandage changes and wound care. Amputation is elected in some dogs to resolve the adverse sequelae associated with infection.31
Pasteurized tumoral autografting
Pasteurized tumoral autografting involves the temporary removal (ostectomy) and pasteurization of the affected bone segment. The treated bone segment is then replaced as an orthotopic autograft and stabilized with a bone plate and screws.18,19 The pasteurization process requires placing the bone segment into a sealed container filled with sterile saline solution preheated to 149 F (65 C).19 The container is kept in a thermostat-controlled water bath and maintained at 149 F for 40 minutes.19 Pasteurization is advantageous in that all the cellular constituents of the excised bone segment are killed, but unlike autoclaving, which is pressurized and done at a higher temperature, important proteins, such as bone morphogenetic proteins, are not damaged.
Ideal candidates for this procedure are dogs with good cortical integrity and without pathologic fractures. Dogs undergoing pasteurized autograft limb salvage have survival times and complication rates comparable to dogs undergoing cortical bone allograft and bone transport osteogenesis limb salvages.18 When this technique was used in conjunction with adjuvant chemotherapy, mean and median survival times in a small cohort of patients were 531 and 324 days, respectively, with overall survival rates of 100%, 50%, and 44% at six, 12, and 18 months, respectively.18 While this technique eliminates the need for establishing and maintaining a bone bank and has the advantage of proper fit to the recipient site, pasteurization increases the duration of surgery, and screw placement in the pasteurized bone segment can be limited by tumor erosion.18
Bone transport osteogenesis
Distraction osteogenesis involves applying gradual traction to certain tissues, which creates stresses that can stimulate and maintain the regeneration of active growth.33 Persistent traction causes tissues to become metabolically activated, resulting in an increase in the proliferative and biosynthetic functions. These processes depend on an adequate blood supply to the tissues being elongated and the stimulating effect of functional weightbearing.33 This biologic phenomenon has been used to control healing and shape bone and soft tissue formation to treat traumatic and pathologic musculoskeletal abnormalities.33
Bone transport osteogenesis is a specific application of distraction osteogenesis. It is used to replace large segmental bone defects and has been used clinically in both animals and people.20,21,29,34-38 This technique involves slowly moving an intercalary segment of healthy normal bone into an adjacent defect. Typically, the intercalary segment of the bone is transported by wires that pass through the bone segment and anchor to a circular external skeletal fixation device. The wires are moved in a linear direction along the fixator frame. As the transport segment is moved, new regenerate bone forms in the trailing distraction pathway. Optimal bone formation is obtained by using a distraction rate of about 1 mm/day.29,36 The regenerate bone acts as a highly vascularized autogenous graft that remodels into lamellar bone. In addition to being successfully used in dogs and people for repairing osseous defects related to trauma and infection,37,38 this technique has been successfully used to resolve subtotal defects of the distal radius and tibia of dogs after tumor resection (Figures 2A & 2B).20,21
Many of the complications associated with cortical bone allografts can be avoided when bone transport osteogenesis is used to replace the resultant bone defect. With autogenous bone produced by bone transport osteogenesis, concerns regarding failure or loosening of internal implant components, graft rejection, transmission of infectious disease, and harbored bacterial infections are either decreased or eliminated.20 Previous research has shown that fewer complications (such as fracture and infection) were associated with the use of bone transport osteogenesis (33%) than with traditional segmental defect replacement (60%).38 The well-vascularized regenerate bone is highly resistant to infection.39
Local recurrence of the tumor is still a concern; however, proper case selection, administration of adjuvant chemotherapy, and adherence to sound surgical oncologic technique help to minimize this potential risk.11,16,20,23,40 While necrosis of the regenerate bone has been reported, this complication may have been related to preoperative radiation administration.20 A recent study has shown that administering cisplatin during the distraction and docking process (the process by which the newly formed and preexisting bone fuse) does not adversely affect regenerate bone formation.41
The principal disadvantage of using bone transport osteogenesis for limb-sparing is the relatively long period that the fixation device must be maintained. This is particularly true when dealing with longer segmental defects. Previously reported applications of bone transport osteogenesis for limb salvage in dogs with osteosarcoma used a rate of 1 mm/day and a rhythm of 0.25 mm/6 hours.20 A large defect may require a substantial amount of time for adequate distraction and consolidation; 100 to 147 days of distraction were reported in cases without local recurrence.20 During this time, weekly visits to the local veterinarian and exercise restrictions such as leash walking are required. A technique known as double bone transport has been recently reported as a treatment for tibial osteosarcoma in dogs.21 This technique involves the simultaneous distraction of two transport segments, allowing a defect to be filled more quickly than with a single transport segment.
Endoprosthesis
A radial endoprosthesis (Veterinary Orthopedic Implants, South Burlington, Vt.) has been developed as an alternative to cortical bone allografting. This prosthesis couples a specially designed limb-sparing bone plate with a metal spacer that spans the defect between the radial osteotomy site and the radiocarpal bone (Figure 3). The articular surface of the radial carpal bone is also removed, and carpal arthrodesis is performed. Advantages of this technique include the lack of the need for a bone bank, less technical difficulty, and a shorter operative time.22 Observations from clinical experience indicate that endoprostheses may be associated with a lower infection rate and that the infections may be more superficial and easier to resolve.22 A theoretical disadvantage is that the host bone is not incorporated into the endoprosthesis. This disadvantage maybe negated, however, by the relatively short survival times typical of most dogs affected with osteosarcoma. In addition, such concerns are not unique to endoprostheses because there is also some question as to whether cortical allografts are effectively incorporated and replaced by recipient bone as well.

FIGURE 3. A photograph of the cranial aspect of a dog forelimb during a radial endoprosthesis limb-sparing procedure (left) and a postoperative lateral radiograph of the forelimb (right).
RADIATION THERAPY
Radiation therapy has been used extensively to treat appendicular osteosarcoma.31,42 Radiation therapy administered before surgery, when given in moderate doses (32 Gy), has been shown to significantly reduce the local tumor recurrence rate after cortical allograft limb-sparing surgery.42 Previous studies, however, have shown that preoperative radiotherapy at higher doses (36 to 52 Gy) causes unacceptable rates of fixation device failure and graft complications.31 As survival is often limited by distant metastasis rather than local recurrence, preoperative radiation has not been shown to increase long-term survival.
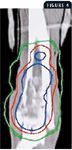
FIGURE 4. A computed tomography image in the dorsal plane of an osteosarcoma in a dog distal tibia. The stereotactic radiosurgery treatment plan is superimposed, and the dose distribution is represented by isodose lines. The image represents only a single slice of the treatment plan. The 75% isodose line (30 Gy) is shown in blue, the 50% isodose line (20 Gy) in red, and the 25% isodose line (10 Gy) in green. Although they appear as lines on the two-dimensional display, isodose lines represent three-dimensional dose shells surrounding the target volume. A transosseous Kirschner wire and fiberglass cast material (used temporarily to anchor the radiation targeting device to the bone) are visible in the top portion of the image.42
Palliative radiation therapy is another alternative to amputation and limb-salvage procedures. This treatment can effectively palliate the pain associated with appendicular osteosarcoma.43-45 Previously described palliative protocols involve three fractions of 10 Gy given at Days 0, 7, and 21.43,44 A more effective protocol has recently been described that uses four 8-Gy fractions with one fraction given every seventh day.45 This new protocol eliminates the two-week gap between the seventh and 21st day and has been shown to provide a higher response rate and longer survival times.45 Using the four-fraction protocol resulted in a 92%45 response rate vs. 74%44 or 80%43 reported using the three-fraction protocol. The median duration of response using the four-fraction protocol is 95 days, with a median survival time of 313 days45—much higher than that observed using the previously described protocols.43,44
Recently, stereotactic radiosurgery has been used to treat dogs with appendicular osteosarcoma.46 Unlike fractional therapy, stereotactic radiosurgery delivers the entire radiation treatment (about 30 Gy) through a single, large dose in a highly targeted manner. The precise nature of stereotactic radiosurgery allows the delivery of a radiation dose that is conformed to the shape of the tumor target, and damage to the normal surrounding tissues is minimized by a steep dose gradient (Figure 4).47 Advantages of administering a single, large fraction include fewer anesthetic episodes and possibly a greater biologic effect on tumor tissue when compared with an equivalent total dose delivered in multiple (e.g. three 10 Gy) fractions.48
Preliminary results with stereotactic radiosurgery in lower extremity (radius and tibia) tumors have shown the ability to provide long-term local control (> 2 years), especially when it is possible to surround the entire tumor with the 30-Gy isodose line during treatment planning and when combined with chemotherapy.46 Currently, carboplatin (300 mg/m2) is given intravenously just before stereotactic radiosurgery as a radiation sensitizer, and carboplatin alone or in combination with doxorubicin is given adjunctively during the convalescent period. Upper extremity (humerus and femur) tumors have also been treated by one of the authors (Farese JP: Unpublished data; currently being evaluated for response) and may become the ideal application of stereotactic radiosurgery since limb-sparing surgery is not performed routinely in these tumor locations. The prominent muscle mass between the tumor and the skin in upper extremity locations enables even larger radiation doses to be used without causing marked radiation skin injury.44
As with conventional radiation therapy, pathologic fracture may occur after treatment since the quality of the bone structure is often compromised by tumor-associated osteolysis. The limiting factors of stereotactic radiosurgery therapy for appendicular osteosarcoma are the size of the tumor and the condition of the bone at the time of therapy, as adequate coverage of large tumors with the 30-Gy isodose line is not always possible and the risk of pathologic fracture remains after treatment. The use of external coaptation to decrease this risk has not been evaluated. Thus, stereotactic radiosurgery should ideally be used to treat appendicular osteosarcomas that are relatively small and have caused minimal bone destruction.
An additional limb-sparing technique that uses intraoperative radiotherapy has been used in a small number of dogs with osteosarcoma located at sites other than the distal radius or ulna.1 After surgically isolating the tumor from the surrounding soft tissues, the surgeon performs an osteotomy through an unaffected portion of the bone proximal or distal to the tumor site. While maintaining joint capsule attachments, the surgeon then rotates the neoplastic bone segment out of the surgical field, and a single dose of 70 Gy is delivered to the neoplastic bone segment. The irradiated bone segment is replaced in situ, and the osteotomy is stabilized with internal fixation.1
CHEMOTHERAPY
Chemotherapy has been used primarily as an adjunct to surgery to help control metastasis. Previous reports have shown that survival time in dogs with appendicular osteosarcoma can be improved by using adjuvant chemotherapy. Reported median survival times are outlined in Table 1.8,16,32,40,49-58 Cisplatin has been shown to prolong the disease-free interval in dogs and remains a commonly used chemotherapeutic agent for treating dogs with osteosarcoma.41 Myelosuppression and nephrotoxicity are the most common side effects noted with cisplatin therapy.59
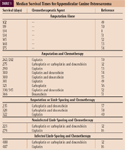
TABLE 1 Median Survival Times for Appendicular Canine Osteosarcoma.
Using cisplatin or doxorubicin as a single agent as adjunctive treatment to amputation has yielded median survival times ranging from 262 days50 to 366 days,51 one-year survival rates ranging from 37%50,54 to 46%,41 and two-year survival rates ranging from 16%50 to 26%.54 These results show a significant improvement in survival over amputation alone, which has been reported to yield a median survival time of 102 to 175 days, a one-year survival rate of 12%, and a two-year survival rate of 2%.8,49,54 Carboplatin is an alternative chemotherapeutic agent that is at least as efficacious as cisplatin.56 Carboplatin is a second-generation platinum compound that, unlike cisplatin, does not induce nephrotoxicity and is easier to administer. Although initially some combination protocols (e.g. cisplatin and doxorubicin) appeared to offer survival benefit over single-agent therapy,60 recent evidence indicates that combination protocols do not increase survival.55,57,58
Other chemotherapeutic agents that have been evaluated for treating canine osteosarcoma in combination with amputation and limb salvage include cisplatin-impregnated open-cell polylactic acid polymer (OPLA-Pt) and liposome-encapsulated muramyl tripeptide-phosphatidylethanolamine (liposome/MTP-PE).61,62 Cisplatin-impregnated OPLA-Pt has been placed in the wound bed adjacent to allografts in limb salvage cases to reduce local tumor recurrence and has been reported to reduce local recurrence by 10%.61 Liposome/MTP-PE has also been shown to be effective in treating dogs with osteosarcoma.62 In one study, dogs treated by amputation and intravenous liposome/MTP-PE had a median survival time of 222 days, while those treated with empty liposomes had a median survival time of only 77 days.62 Aside from mild elevations in body temperature (1.8 to 3.6 F [1 to 2 C]) for two to six hours after injection, treatment with liposome/MTP-PE was well-tolerated.
Chemotherapy has also been evaluated in dogs with measurable pulmonary metastatic osteosarcoma.63 Single-agent therapy with either cisplatin, doxorubicin, or mitoxantrone was ineffective in treating measurable metastatic disease, providing a median survival time of only 61 days (range, 14 to 192 days).63
MEDICAL MANAGEMENT
Many owners of affected dogs do not perceive the outcome of surgery (with or without chemotherapy) to be worthwhile. In these instances, medical management of the primary tumor may be elected. Medical management typically consists of daily treatment with a nonsteroidal anti-inflammatory drug (NSAID) to alleviate the associated bone pain. When lameness worsens and is refractory to treatment with NSAIDs, oral formulations of various opioids may provide more effective pain control.
In human medicine, osteolytic bone diseases (e.g. bone metastases and hypercalcemia of malignancy) are often treated with a class of drugs called bisphosphonates.64 Bisphosphonates reduce bone resorption by inhibiting osteoclast function.64 There is only one clinical report of the use of bisphosphonates in dogs with spontaneously occurring osteosarcomas.65 In this report, alendronate was used as palliative treatment in two cases of primary canine osteosarcoma, one affecting the tibia and the other affecting the maxilla. According to the report, both dogs remained comfortable, surviving for 12 and 10 months, respectively. However, since only two dogs were studied, there was no control group, and survival was the only parameter measured, it is difficult to conclude what benefit alendronate provided. This case report, along with evidence for efficacy in the treatment of various malignant bone diseases in people, has prompted veterinary clinicians to treat some cases of primary canine osteosarcoma with bisphosphonates. Recently, the bisphosphonates alendronate, pamidronate, and zoledronate have been shown to inhibit canine osteosarcoma cell growth in vitro, raising the possibility that bisphosphonates may also be helpful as adjunctive chemotherapeutic agents.66-68
CONCLUSION
Amputation in conjunction with chemotherapy continues to be the most common and effective treatment available for dogs with appendicular osteosarcoma5,41; however, new approaches are being developed that should prolong survival times and decrease morbidity. As the efficacy of treatments such as limb-salvage surgeries, radiation, and chemotherapy are established, practitioner and owner interest will grow.
Editors' Note: One of the authors, Dr. Charles A. Kuntz, developed the endoprosthesis method discussed above and receives compensation for every unit sold from Veterinary Orthopedic Implants, which manufactures the endoprostheses.
Carl T. Jehn, DVM
James P. Farese, DVM, DACVS
Daniel D. Lewis, DVM, DACVS
Department of Small Animal Clinical Sciences and the Center for Veterinary Sports Medicine
College of Veterinary Medicine
University of Florida
Gainesville, FL 32610-0126.
Nicole Ehrhart, VMD, MS, DACVS
The Animal Cancer Center
College of Veterinary Medicine
Colorado State University
Fort Collins, CO 80523.
Charles A. Kuntz, DVM, MS, DACVS
Regional Veterinary Referral Center
6651-F Backlick Road
Springfield, VA 22150
REFERENCES
1. Withrow SJ, MacEwen EG. Tumors of the skeletal system. In: Clinical veterinary oncology. Philadelphia, Pa: JB Lipincott Co, 1989;378-392.
2. Priester WA. The occurrence of tumors in domestic animals. In: National Cancer Institute, monograph no. 54. NIH publication no. 80-2046. New York: Oxford University Press, 1982.
3. Tjalma RA. Canine bone sarcoma: Estimation of relative risk as a function of body size. J Natl CancerInst 1966;36:1137-1150.
4. Brodey RS, Riser WH. Canine osteosarcoma: A clinicopathologic study of 194 cases. Clin. Orthop. 1969;62:54-64.
5. Brodey RS, Abt DA. Results of surgical treatment in 65 dogs with osteosarcoma. J Am Vet Med Assoc 1976;168:1032-1035.
6. Ling GV, Morgan JP, Pool RR. Primary bone tumors in the dog: A combined clinical, radiographic, and histologic approach to early diagnosis. J Am Vet Med Assoc 1974;165:55-67.
7. Phillips L, Hager D, Parker R, et al. Osteosarcoma with a pathologic fracture in a six-month-old dog. Vet Radiol 1986;27:18-19.
8. Spodnick GJ, Berg J, Rand WM, et al. Prognosis for dogs with appendicular osteosarcoma treated by amputation alone: 162 cases (1978-1988). J Am Vet Med Assoc 1992;200:995-999.
9. McGavin MD, Carlton WW, Zachary JF. Diseases of bone. In: Thomson's special veterinary pathology. St. Louis, Mo: Mosby Inc 2001;523-524.
10. Heyman SJ, Diefenderfer DL, Goldschmidt MH, et al. Canine axial osteosarcoma: A retrospective study of 116 cases (1986-1989). Vet Surg 1992;21:304-310.
11. Straw RC, Withrow SJ. Limb-sparing surgery versus amputation for dogs with bone tumors. Vet Clin North Am Small Anim Pract 1996;26:135-143.
12. Kirpensteijn J, van den Bos R, Endenburg N. Adaptation of dogs to the amputation of a limb and their owners' satisfaction with the procedure. Vet Rec 1999;144:115-118.
13. Withrow SJ, Hirsch VM. Owner response to amputation of a pet's leg. Vet Med Small Anim Clin 1979;74:332-334.
14. Carberry CA, Harvey HJ. Owner satisfaction with limb amputation in dogs and cats. J Am Anim Hosp Assoc 1987;23:227-232.
15. Budsberg SC. Amputations. In: Olmstead ML, ed. Small animal orthopedics. Philadelphia, Pa: WB Saunders 1995;531-548.
16. O'Brien MG, Straw RC, Withrow SJ. Recent advances in the treatment of canine appendicular osteosarcoma. Compend Cont Ed Pract Vet 1993;15:939-946.
17. LaRue SM, Withrow SJ, Powers BE, et al. Limb-sparing treatment for osteosarcoma in dogs. J Am Vet Med Assoc 1989;195:1734-1744.
18. Morello E, Vasconi E, Martano M, et al. Pasteurized tumoral autograft and adjuvant chemotherapy for the treatment of canine distal radial osteosarcoma: 13 cases. Vet Surg 2003;32:539-544.
19. Buracco P, Morello E, Martano M, et al. Pasteurized tumoral autograft as a novel procedure of limb sparing in dogs: A clinical report in a canine distal radial osteosarcoma. Vet Surg 2002;31:525-532.
20. Tommasini Degna M, Ehrhart N, Feretti A, et al. Bone transport osteogenesis for limb salvage following resection of primary bone tumors: Experience with six cases (1991-1996). Vet Comp Orthop Traumatol 2000;13:18-22.
21. Rovesti GL, Bascucci M, Schmidt K, et al. Limb sparing using a double bone-transport technique for treatment of a distal tibial osteosarcoma in a dog. Vet Surg 2002;31:70-77.
22. Liptak JM, Dernell WS, Ehrhart N, et al. Canine appendicular osteosarcoma: Curative-intent treatment. Compend Cont Ed Pract Vet 2004;26:186-196.
23. Gilson SD, Stone EA. Principles of oncologic surgery. Compend Cont Ed Pract Vet 1990;12:827-839.
24. Enneking WF, Spanier SS, Goodman MA. A system for surgical staging of musculoskeletal sarcoma. 1980. Clin Orthop 2003;415:4-18.
25. Powers BE, LaRue SM, Withrow SJ, et al. Jamshidi needle biopsy for diagnosis of bone lesions in small animals. J Am Vet Med Assoc 1988;193:205-210.
26. Vasseur P. Limb preservation in dogs with primary bone tumors. Vet Clin North Am Small Anim Pract 1987;17:889-903.
27. Scully SP, Temple HT, O'Keefe RJ, et al. The surgical treatment of patients with osteosarcoma who sustain a pathologic fracture. Clin Orthop 1996;324:227-232.
28. Dick HM, Strauch RJ. Infection of massive bone allografts. Clin Orthop 1994;306:46-53.
29. Aronson J. Limb-lengthening, skeletal reconstruction, and bone transport with the Ilizarov method. J Bone Joint Surg Am 1997;79:1243-1258.
30. Dernell WS, Withrow SJ, Straw RC, et al. Clinical response to antibiotic impregnated polymethyl methacrylate bead implantation of dogs with severe infections after limb sparing and allograft replacement-18 cases (1994-1996). Vet Comp Orthop Traumatol 1998;11:94-99.
31. Thrall DE, Withrow SJ, Powers BE, et al. Radiotherapy prior to cortical allograft limb sparing in dogs with osteosarcoma: A dose response assay. Int J RadiatOncol Biol Phys 1990;18:1351-1357.
32. Liptak JM, Dernell WS, Lascelles BDX, et al. Survival analysis of dogs with appendicular osteosarcoma treated with limb-sparing surgery and adjuvant carboplatin or carboplatin and doxorubicin, in Proceedings. 21st Annu Vet Cancer Soc Conf 2001;39.
33. Ilizarov GA. The tension-stress effect on the genesis and growth of tissues. In: The Transosseous osteosynthesis. Berlin, Germany: Springer-Verlag, 1992;137-255.
34. Aronson J. Cavitary osteomyelitis treated by fragmentary cortical bone transportation. Clin Orthop 1992;280:153-159.
35. Villa A. Isolated radial lengthening. In: Bianchi Maiocchi A, ed. Advances in Ilizarov apparatus assembly. Milan, Italy: Medicalplastic srl, 1985;90.
36. Welch RD, Lewis DD. Distraction osteogenesis. Vet Clin North Am Small Anim Pract 1999;29:1187-1205.
37. Cierny G 3rd, Zorn KE. Segmental tibial defects. Comparing conventional and Ilizarov methodologies. Clin Orthop 1994;301:118-123.
38. Cattaneo R, Catagni M, Johnson EE. The treatment of infected nonunions and segmental defects of the tibia by the methods of Ilizarov. Clin Orthop 1992;280:143-152.
39. Minematsu K, Tsuchiya H, Taki J, et al. Blood flow measurement during distraction osteogenesis. Clin Orthop 1998;347:229-235.
40. Berg J, Weinstein MJ, Schelling SH, et al. Treatment of dogs with osteosarcoma by administration of cisplatin after amputation or limb-sparing surgery: 22 cases (1987-1990). J Am Vet Med Assoc 1992;200:2005-2008.
41. Ehrhart N, Eurell JA, Tommasini M, et al. Effect of cisplatin on bone transport osteogenesis in dogs. Am J Vet Res 2002;63:703-711.
42. Withrow SJ, Thrall DE, Straw RC, et al. Intra-arterial cisplatin with or without radiation in limb-sparing for canine osteosarcoma. Cancer 1993;71:2484-2490.
43. Ramirez O 3rd, Dodge RK, Page RL, et al. Palliative radiotherapy of appendicular osteosarcoma in 95 dogs. Vet RadiolUltrasound 1999;40:517-522.
44. McEntee MC, Page RC, Novotney CA, et al. Palliative radiotherapy for canine appendicular osteosarcoma. Vet RadiolUltrasound 1993;34:367-370.
45. Green EM, Adams WM, Forrest LJ. Four fraction palliative radiotherapy for osteosarcoma in 24 dogs. J Am Anim Hosp Assoc 2002;38:445-451.
46. Farese JP, Milner R, Thompson MS, et al. Stereotactic radiosurgery for the treatment of osteosarcomas involving the distal portions of the limbs in dogs. J Am Vet Med Assoc 2004;225:1567-1572.
47. Bova FJ, Buatti JM, Friedman WA, et al. The University of Florida frameless high-precision stereotactic radiotherapy system. Int J Radiat Oncol Biol Phys 1997;38:875-882.
48. Fowler JF. The linear-quadratic formula and progress in fractionated radiotherapy. Br J Radiol 1989;62:679-694.
49. Shapiro W, Fossum TW, Kitchell BE, et al. Use of cisplatin for treatment of appendicular osteosarcoma in dogs. J Am Vet Med Assoc 1988;192:507-511.
50. Straw RC, Withrow SJ, Richter SL, et al. Amputation and cisplatin for treatment of canine osteosarcoma. J Vet Intern Med 1991;5:205-210.
51. Berg J, Weinstein MJ, Springfield DS, et al. Results of surgery and doxorubicin chemotherapy in dogs with osteosarcoma. J Am Vet Med Assoc 1995;206:1555-1560.
52. Berg J, Gebhardt MC, Rand WM. Effect of timing of postoperative chemotherapy on survival of dogs with osteosarcoma. Cancer 1997;79:1343-1350.
53. Thompson JP, Fugent MJ. Evaluation of survival times after limb amputation, with and without subsequent administration of cisplatin, for treatment of appendicular osteosarcoma in dogs: 30 cases (1979-1990). J Am Vet Med Assoc 1992;200:531-533.
54. Mauldin GN, Matus RE, Withrow SJ, et al. Canine osteosarcoma: Treatment by amputation versus amputation and adjuvant chemotherapy using doxorubicin and cisplatin. J Vet Intern Med 1988;2:177-180.
55. Chun R, Garrett LD, Henry C, et al. Cisplatin and doxorubicin combination for the treatment of canine osteosarcoma, in Proceedings. 21st Annu Vet Cancer Soc Conf 2001;7.
56. Bergman PJ, MacEwen EG, Kurzman ID, et al. Amputation and carboplatin for treatment of dogs with osteosarcoma: 48 cases (1991 to 1993). J Vet Intern Med 1996;10:76-81.
57. Bailey D, Erb H, Williams L, et al. Carboplatin and doxorubicin combination chemotherapy for the treatment of appendicular osteosarcoma in the dog. J Vet Intern Med 2003;17:199-205.
58. Kent MS, Strom A, London CA, et al. Alternating carboplatin and doxorubicin as adjunctive chemotherapy to amputation or limb-sparing surgery in the treatment of appendicular osteosarcoma in dogs. J Vet Intern Med 2004;18:540-544.
59. Ogilvie GK, Straw RC, Powers BE, et al. Prevalence of nephrotoxicosis associated with a short-term saline solution diuresis protocol for the administration of cisplatin to dogs with malignant tumors: 61 cases (1987-1989). J Am Vet Med Assoc 1991;199:613-616.
60. Chun R, Kurzman ID, Couto CG, et al. Cisplatin and doxorubicin combination chemotherapy for the treatment of canine osteosarcoma: A pilot study. J Vet Intern Med 2000;14:495-498.
61. Straw RC, Withrow SJ, Douple EB, et al. Effects of cis-diamminedichloroplatinum II released from D,L-polylactic acid implanted adjacent to cortical allografts in dogs. J Orthop Res 1994;12:871-877.
62. MacEwen EG, Kurzman ID, Rosenthal RC, et al. Therapy for osteosarcoma in dogs with intravenous injection of liposome-encapsulated muramyl tripeptide. J Natl Cancer Inst 1989;81:935-938.
63. Ogilvie GK, Straw RC, Jameson VJ, et al. Evaluation of single-agent chemotherapy for treatment of clinically evident osteosarcoma metastases in dogs: 45 cases (1987-1991). J Am Vet Med Assoc 1993;202:304-306.
64. Ross JR, Saunders Y, Edmonds PM, et al. Systematic review of role of bisphosphonates on skeletal morbidity in metastatic cancer. BMJ 2003;327(7413):469.
65. Tomlin JL, Sturgeon C, Pead MJ, et al. Use of the bisphosphonate drug alendronate for palliative management of osteosarcomas in two dogs. Vet Rec 2000;147:129-132.
66. Farese JP, Ashton J, Milner R, et al. The effect of the bisphosphonate alendronate on viability of canine osteosarcoma cells in vitro. In Vitro Cell Dev Biol Anim 2004;40:113-117.
67. Poirier VJ, Huelsmeyer MK, Kurzman ID, et al. The bisphosphonates alendronate and zoledronate are inhibitors of canine and human osteosarcoma cell growth in vitro. Vet Compar Oncol 2003;1:207-215.
68. Ashton J, Farese JP, Milner RJ, et al. Investigation of the effect of pamidronate disodium on the in vitro viability of osteosarcoma cells from dogs. Am J Vet Res 2005:in press.