Dermatology for technicians (Proceedings)
Protocols are useful in helping to diagnose and treated many different disorders. Part of any good protocol should be a minimum data base (MDB). In addition to signalment, history, etc in veterinary dermatology laboratory testing should be a component of this data base.
Protocols are useful in helping to diagnose and treated many different disorders. Part of any good protocol should be a minimum data base (MDB). In addition to signalment, history, etc in veterinary dermatology laboratory testing should be a component of this data base. Just as you may have a standard set of tests for diarrhea you should have a standard set of tests for dermatology cases. In a general practice you should be performing these tests MULTIPLE times daily. In addition to these tests other commonly, easily performed tests are
1. Skin scrapings **
2. Impressions smears **
3. Ear cytologies ** if ear disease is present
4. Fine tooth combing **
5. Fine needle aspirate
6. Hair plucks/trichograms
7. Woods lamp and fungal culture
8. Bacterial culture
9. CBC, serum chemistry profile and urinalysis
10. Adrenal function tests
11. Thyroid profile
12. Food trial
13. Intradermal testing (or serum testing) and allergen specific immunotherapy
** Component of MDB
Slide Examination
When examining a specimen microscopically, once a sample is collected (+/- processed- see cytology) the entire slide should first be examined under a low (scanning) power (4X objective). Use this scan to evaluate the quality and quantity of the sample collected, to find larger mites (eg Sarcoptes, Cheyletiella) and to identify areas that should be examined more closely. After evaluating the slide under 4x power, the slide should be examined using low power (10X objective) for smaller mites (eg Demodex) and then oil immersion (100X) to identify organisms (eg bacteria, yeast) and cells (eg neutrophils, eosinophils, keratinocytes, neoplastic cells etc). With experience you will be able to identify cells w/the 40X objective, thereby saving time. You can improve clarity on 40X by placing immersion oil on the slide and then covering the area w/a cover slip. You then can examine it w/the 40X. When you are looking for cells or organisms raise the condenser but when examining for ectoparasites, if you drop the condenser you increase the contrast making it easier to identify the parasite.
Skin scraping
Let's begin with the MBD- before performing skin scrapings you should ask the following questions
1. What technique do I do (broad superficial or deep scrapings or both)
2. Where do I need to skin scrape?
3. What lesions am I looking for to scrape?
The answers to these questions depend on which parasite you suspect. If you suspect a superficial mite (Sarcoptes, Notoedres, Demodex gatoi (cats), Demodex cornei (dogs) Cheyletiella) then broad superficial scrapings should be performed. Deep skin scrapings should be performed when Demodex canis or cati is suspected. (Table 1)
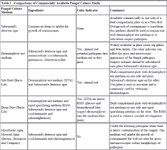
Table 1 - Comparisons of Commercially Available Fungal Culture Media
When performing superficial scrapes be sure to:
• Scrape from appropriate areas. For Sarcoptes you will be more successful if you scrape pinnal edges, the elbows, ventral chest and hocks. In addition any papular or erythematous lesion should be scraped.
• For any of the superficial mites, broad scraping should be performed. Remember that mites associated w/hypersensitivity (eg Sarcoptes, Cheyletiellai) may be difficult to find due to their low numbers so be sure to take multiple (10-15) sites. In contrast to demodex, all scrapes can be placed on 2 or 3 slides because the quantity of mites present is not important, they are either found or not.
When performing a deep skin scrape for demodex (this applies to mostly to dogs) there are a few pitfalls to avoid. By avoiding these errors the diagnosis and your management of demodex will improve.
These include:
• Failing to squeeze the skin prior to scraping. This helps express the Demodex from the hair follicles
• Failing to record location of scrapes;
• Failing to record numbers & stages present;
• Failing to record whether the mites are alive or dead;
• Failing to clip hair at skin scrapings sites (if it is a recheck appointment, the hair may be regrowing preventing proper sample collection);
• Failure to squeeze the skin prior to scraping to try
• Failure to recognize that lesions that are granulomatous & fibrotic, especially on the paws may have demodex that are hard to demonstrate on skin scrapings and a skin biopsy may be necessary to diagnosis;
• Failure to sedate dogs if the feet are to be scraped
• Failing to scrape hyperpigmented areas even if they are not alopecic;
• Failing to scrape areas with comedones even if they are not alopecic
• Failing to scrape if a dog only has greasy seborrhea (especially along the dorsum). A long body type of demodex mite has been identified (Demodex injai). This mite lives in the sebaceous glands of the dog's skin, and thus, is commonly associated with "greasy coats" rather than the moth eaten or pustular appearance that we are used to seeing.
• Failing to take broad superficial skin scrapes even if demodex is the only parasite you suspect. There is a short bodied demodex mite (Demodex cornei), which lives on the surface of the skin layer. Note that there may be a low number of these mites found because of the superficial location of the mites allowing removal by the animal.
Cytology
Cytologic examination is another very commonly performed procedure in dermatology that should be performed on any dog or cat presented w/skin or ear disease. Cytology is used to identify the presence (and/or type) of:
• Bacterial or fungal organisms (Malassezia);
• Neoplastic cells;
• Inflammatory cells;
• Abnormal cells (eg acantholytic keratinocytes associated w/pemphigus foliaceus)
• Both the direct smear (scrapings, impression, roll smear or tape) and fine needle aspirates (FNA) are valuable techniques that need to be mastered.
When the skin is scaly, a superficial skin scraping can be useful. A very small amount of mineral oil is placed on a #15 scalpel blade to help keep the scale on the blade once it has been collected. The lesion is scraped a few times, and the material collected is placed on a microscope slide, stained (see below about staining samples), and examined microscopically at 40X and 100X.
Direct smears can be collected by a variety of ways.
Impression (touch) smears are useful when there is an erosion, ulcer, crust, moist or greasy lesion. To perform an impression smear, a slide is firmly applied to a lesion and, in most cases, is then gently moved back and forth a few times to increase the yield. Clear acetate tape ("scotch tape") is also useful for collecting samples. Some people will use slides that are "sticky" on one side. These slides are reported to increase the yield of sample collected but the author finds that a standard slide works quite well. The slide is then processed and examined as previously described
If the lesion is fluid filled (eg pustule, papule) but is too small for a fine needle aspirate, "lance" the lesion with a 25 gauge needle and then do an impression smear.
When sampling crusts, lift the crust and rub both the underside of the crust and the surface of the skin.
Roll smears (swabs) are used when it would be difficult to get a slide into the affected area. This could be the face fold, the interdigital space on cats and small dogs and the ear canals in all dogs and cats. A cotton tipped applicator is gently rubbed back and forth across the lesion and then the material from the applicator stick is rolled back and forth on the slide. If the lesion is scaly, applying a small amount of mineral oil to the swab can help with collection. The sample is rolled onto a microscope slide, stained and examined as previously described.
A fine needle aspirate is performed when a solid or fluid filled mass or lesion is present. A 22-25 gauge needle attached to a 12 cc syringe is placed into the lesion and suction is applied by pulling back the plunger of the syringe (½ to ¾ of the way). The syringe plunger is pulled back and released a few times. Don't aspirate aggressively enough that you get blood contaminating the sample (you should not see blood in the hub of the needle). After aspirating one spot, stop aspirating and redirect the needle in the mass w/o pulling out and repeat the aspiration. This can be repeated 2 or 3 times on each sampling attempt. The needle is disconnected from the syringe, the syringe is filled w/air and the needle is placed back on the syringe. The material is then ejected from the needle by compressing the plunger. If the lesion is a fluid filled you only have to pull back far enough to get a sample into the syringe. Note- Measuring and noting the location of the masses is valuable for monitoring treatment.
Regardless of the collection technique (except when using the tape prep) historically the author would heat fix the sample, using a cigarette lighter, and then wait a minute or so to allow it to cool. The slide was then stained w/a modified Wright stain (Diff Quik®). There are 3 jars in the Diff Quik® kit. The first jar is a fixative containing methanol, the second contains buffered xanthene dye, which stains the cells and organisms red and the third contains a buffered thiazine dye (methylene blue) which stains the cells and organisms purple. After drying the slide would then be examined.
More recently I have bypassed both the fixative step and the second step (eosin) and directly go to the 3rd step using the methylene blue only. It doesn't appear to hinder the identification of organisms or inflammatory cells. If using the tape prep I will put a drop on stain on the slide and then place the tape, sticky side down, over the stain and examine.
Ear cytologies are performed to identify mites, infectious agents, ectoparasites and inflammatory cells. A cotton tip applicator is used to collect the samples prior to instituting therapy. Results of the cytology help direct appropriate therapy (presence of infectious agents would indicate the need for antimicrobial therapy). I will also perform ear cytologies during therapy if either the ear(s) are not responding to treatment OR if there were mostly rods on the initial cytology even if the ear(s) look normal at recheck.
Regardless of how the sample is collected to examine the slide involves the following steps:
1. Raise the condenser and have the light on the highest level
2. Scan the slide slowly at 10 x to identify areas with the most # of cells
3. Use 100X to ID bacteria (cocci/rods), Malassezia, inflammatory cells and neoplastic cells
a. With practice you can use 40X to ID Malassezia and inflammatory cells
4. Look at several representative fields and record your findings
a. For skin cytologies
1. For bacteria look in 10 fields and record a range (eg 0-5, 5-10, 10-20 etc) – be sure to note if they are cocci or rods and whether they are intracellular or extracellular
2. For Malassezia look in 20-25 fields (unless they are ID sooner). Report them as negative/+0 if NO Malassezia is found, +1 if 1 or 2 organisms are found (total #) in all the fields examined and there were never more than 1 in a field, report a +2 if there are more than 1 organism in a field or 1 organism q 3-4 oil fields
b. For ear cytologies
1. There is no universal agreement as to what are normal # of cocci or Malassezia from an ear cytology
a. Depending on the study, cutoff numbers, per oil immersion field (multiple by 2.5 to get per HPF), between normal and abnormal ears range from >1 Malassezia to >4 Malassezia and from >1 cocci to >10 cocci. It is my opinion that the number of organisms needed to be present to be considered significant is not just a "number". I don't perform cytology on normal ears – I only do them if the ears are inflamed or have exudate. In that case ANY organism seen will be treated as part of the therapy regardless of the number present
2. Inflammatory cells or rod shaped bacteria are never present in a normal ear.
Fine tooth combing
Combing of the hair with a fine tooth comb ("flea comb") is a method that can be useful in finding fleas and other ectoparasites (ticks, lice and Cheyletiella). You may also detect miliary lesions on cats that were not appreciated on your physical examination.
Trichogram ("hair plucks")
Veterinarians are frequently presented w/animals that have hair loss. In establishing the diagnosis of the hair disease, signalment, history (constitutional signs?) and physical examination (eg pot belly?) are all important components in establishing a diagnosis. There are times that even w/this information the cause of the alopecia has not been established. A trichogram, which is a microscopic evaluation of plucked hairs, may be a useful tool to help identify the underlying cause.
To perform a hair pluck
• Grab the hair that is to be examined w/forceps.
• Pluck the hairs gently at the base and roll your wrist to gently remove the hair
• Place the hairs in mineral oil on a slide w/the hairs running in the same direction.
• Apply a coverslip
• Examine microscopically
If the alopecia is post traumatic (pruritus) or due to fragile hairs (eg dermatophytosis) the distal end of the hairs will be broken. If the hair loss is spontaneous (eg endocrinopathy) the tips are tapered.
Hair plucks can also be useful in ruling in (but not ruling out) demodicosis. Other ectoparasites may also be identified such as Cheyletiella or lice.
Follicular cast can also be identified w/hair plucks. Follicular casts refers to the accumulation of keratin debris that adheres to the hair shaft as it extends out of the hair follicle. This finding indicates a follicular keratinization disorder which occurs w/vitamin A responsive dermatosis (Cockers), follicular infections (demodex, dermatophyte, bacterial), sebaceous adenitis, endocrinopathy (hyperadrenocorticism, hypothyroidism) or primary seborrhea such as ear margin seborrhea.
Bacterial cultures
In the past bacterial cultures were not frequently performed in dogs with skin disease since Staphylococcus intermedius was the most common bacterial pathogen and had a predictable susceptibility profile. Unfortunately it isn't that simple any more. Staphylococcus intermedius, Staphylococcus pseudintermedius (like Prince, this is the bacteria previously known as Staphylococcus intermedius), Staphylococcus schleiferi subsp. Schleiferi, Staphylococcus schleiferi subsp. coagalens, and Staphylococcus aureus all w/variable susceptibilities (methicillin resistant, multidrug resistant, combination) are now associated w/pyodermas in dogs. Indications for bacterial culture and susceptibility testing in the dog or cat have therefore been modified. This would include the presence of:
• Nodules;
• Deep draining tracts;
• A bacterial infection of the skin (confirmed by identifying intracellular bacteria and degenerative neutrophils) that fails to respond to appropriate antibiotic therapy;
A bacterial infection of the skin that is failing to respond to appropriate antibacterial therapy (not recurrent but failure to respond) especially if there are immunosuppressed individuals in the house hold
• Suspicion of an uncommon bacterial infection (atypical mycobacteria, nocardia, actinobacillus);
• Suspicion of an anaerobic infection (gas pocket formation);
A few tips:
• Mini-Tip Culturette (Becton Dickinson Microbiology Systems) - These are culture swabs w/very small tips allowing insertion into the lesion. Also by limiting the size of the tip, a more precise sample can be collected.
• Taking samples from 2 or 3 lesions if possible will increase the likelihood of identifying all pathogens
• Do cytology concurrently
• When selecting a lesion to culture – from best to worse – pustule >papule>crust>epidermal collarette
• If you are sampling a crust- lift the crust and swab w/the culturettes the underside of the crust and the surface of the skin under the crusts
• For an epidermal collarette lift the edge of the collarette- if you are not able to do this then clip the hair w/scissors to expose the collarette and take a the culturette swab and gently roll it across the collarette 3 to 4 times.
• Have the lab do tube dilution (MIC) rather than disc diffusion (Kirby-Bauer)
• Gentle cleansing with an alcohol swab will help remove surface debris. Once the lesion is dry, a sterile 20 gauge needle can be used to lance the pustule/papule or used to lift the crust. Don't use alcohol if the lesion you are culturing is a collarette
Wood's lamp examination and fungal culture for dermatophytes
Dermatophyte infection is a common problem in cats and young animals. Proper collection of the specimen is critical in identifying this infection. The first step is to examine the animal with a Wood's lamp. You should let the Wood's lamp warm up for at least 10 minutes, and then shine the light on the hair coat looking for apple-green glow to the entire hair shaft. Remember all crusts will glow and some topical medications will too. A positive test is suggestive of dermatophytes, but you need to culture to confirm this. Please note that a negative test does not rule out dermatophytosis, in fact you should only use the lamp to guide in selecting hairs to pluck for culture.
If the Wood's lamp is negative, and there are focal lesions, the lesions should be gently cleansed with alcohol reduce surface bacterial contamination. Using a clean hemostat, pluck hairs near the base so that you can get the hairs close to the bulb. Also scrape a small amount of scale/crust from the edge of the lesions. This will increase the success rate of identifying dermatophyte infections. If there are diffuse lesions or you are screening a cat for infection, a Mackenzie toothbrush method is used.
If the Wood's lamp is positive you should select the glowing hairs for inoculation of your culture.
Once a sample is collected press the specimen onto the surface of the media, close cover and place culture plate in a darkened area (see below for more details)
Culture plates (table 1). I use a split plate that has Sabouraud's dextrose agar (SDA) on one ½ and dermatophyte test media on the other half. DTM contains Sabouraud's dextrose agar w/cyclohexidime (antifungal), gentamicin and chlorotetracycline (antibacterial). It also contains phenol red (pH indicator). SD agar is better than DTM because it allows better growth of macroconidia and won't mask colony pigmentation but because it is a general fungal medium it allows many different fungi to grow other than just dermatophytes. The DTM is useful because it helps identify dermatophytes more easily because of the medium color change and also the antifungal and antibacterial agents added eliminate many other organisms that grow on SDA. This product that contains both medium is the "best of both worlds". Microscopic examination of the macroconidia along with colony pigmentation can aid in the speciation of the dermatophyte. It should be incubated in 30% relative humidity and 75-86O F. You must check the culture DAILY and record the findings. It is important to note when the media changes color w/respect to colony growth. Large amount of growth w/small color change (contaminant) vs. small growth & large color change (dermatophyte). The color of colony is important in determining contaminant vs. dermatophyte, as is microscopic examination of macroconidia. To get the sample for microscopic examination, apply sticky side of clear acetate tape to growth. Then stain the sample with Lactophenol cotton blue

Table 2
Growth characteristics on SDA that are useful in identifying the 3 most common dermatophyte infections in small animals are (see tables 2 and 3):
• Microsporum Canis colonies are white cottony to woolly
• Microsporum gypseum colonies are buff to cinnamon
• Trichophyton mentagrophytes colonies are white to cream color

Table 3
By microscopically examining the sample you can speciate the dermatophyte. By speciating the dermatophyte you can tell the source of the infection (see below).
Macroconidia
• M. Canis –produce many spindle shaped, thick-walled macroconidia with asymmetrical terminal knob on end.
Contains > 6 cells. Cats are the source of this organism.
• M. Gypseum – produces many ellipsoid, thin-walled macroconidia, with symmetrically rounded tip and has less than 6 cells. Soil is the source of this organism.
• Trichophyton mentagrophytes- produce low numbers of cigar shaped macroconidia with thin smooth walls. Rodents are the source of this organism.
Remember zoonotic potential both when you handle these samples and when you dispose of them, you must treat them as biomedical waste.