EPE: A growing veterinary concern in foals
Though its clinical signs can be vague, equine proliferative enteropathy should always be considered in foals with hypoproteinemia.
Next >
EPE is a disease of foals caused by the obligate intracellular organism and gram-negative bacteria L. intracellularis.1 Once in the gut, it resides freely in the apical cytoplasm of infected intestinal enterocytes, resulting in a thickened small, and sometimes large, intestine.1 It mainly affects weanling foals and causes fever, lethargy, peripheral edema, diarrhea, colic and weight loss.
Though many foals are exposed to the organism, only a few typically develop the disease. However, in recent years, reported cases of EPE have been increasing, primarily in post-weanling foals and occasionally in adult horses. The disease has has been reported in the U.S., Canada, Europe, South Africa and Australia.1
“We don't feel that it's an emerging disease anymore,” states Nicola Pusterla, DVM, DACVIM, professor of equine medicine and epidemiology at UC Davis School of Veterinary Medicine. “It's been described since 1986 and clearly recognized since 2000. It's a disease that has essentially reached almost a worldwide distribution.”
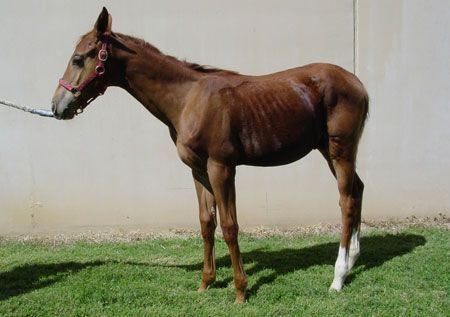
A foal with EPE due to
L. intracellularis
infection shows signs of severe weight loss, a poor coat and lethargy. See page 4 for a photo of this foal after treatment. (Photos courtesy of Dr. Nicola Pusterla)
Incidence
Pusterla notes that EPE typically affects foals less than 1 year of age-and even as young as 3 months of age. But in California, one of the oldest horses that developed EPE was a 3-year-old miniature horse. “We even see it in young 2- to 3-year-old horses at racetracks,” he says.
Pusterla goes on to explain that the disease is a bit strange-it goes from being mainly sporadic with just a single farm having one case a year to farms that are now more endemic. “Even well-managed farms in central Kentucky have about 10 to 15 percent of their foal crop getting sick each year,” he says.
Predisposing factors, such as the stress of weaning and parasitism, have been suggested in the development of EPE in foals.1 It has also been suggested that foals that develop EPE were likely exposed to L. intracellularis via the oral ingestion of infected rabbit feces.2 A recent study has shown that feces from rabbits that were experimentally infected with an equine isolate of L. intracellularis served as infectious material for weanling foals.3 Fecal-oral transmission of L. intracellularis has also been documented in naïve foals housed with critically infected foals experimentally challenged with an equine isolate of L. intracellularis.3
Epidemiological investigations of premises from which clinical cases have been identified have revealed that 10 to 65 percent of unaffected foals and adult horses test seropositive for L. intracellularis.1
Diagnosis
In addition to recognizing the clinical signs, clinicians can make a diagnosis with evidence of hypoproteinemia, thickened segments of the small intestinal wall as seen on abdominal ultrasound, positive serology and molecular detection of L. intracellularis in the feces.
According to Pusterla, one of the main characteristics of the disease is hypoproteinemia due to low serum albumin levels. And since there are not many other diseases causing hypoproteinemia, L. intracellularis infection should always be a consideration with this finding, especially with the presence of GI signs. In EPE cases, the serum protein is generally less than 50g/L and albumin less than 20g/L. A combination of decreased feed intake, coupled with malabsorption and protein-losing enteropathy due to the proliferative nature of the disease, are likely causes for low serum albumin levels.2
In horses, lesions are most commonly seen in the ileum near the ileal-cecal junction and appear as a thickening of the mucosa. Gross lesions are not evident in all cases of EPE and may often be overlooked. The intestines show irregular and patchy subserosal edema. The ileal mucosa is thickened with deep folds, and chronically affected animals may have patches of pseudomembrane covering the mucosa. Hypertrophy and thickening of the muscularis mucosa may occur in chronically affected or recovering animals.3
Treatment and prognosis
Once the disease is diagnosed, the treatment is fairly specific and effective, and with treatment, the outcome is generally good. In one study of 57 foals, the survival rate was 95 percent.4 But in a retrospective assessment of hospital cases in California and central Kentucky, 15 to 20 percent of foals during recent years did not survive.4 It's generally older foals that have complications, like renal failure or GI tract translocation, and become septicemic, says Pusterla.
He recommends a seven- to 10-day course of antibiotic therapy, unless there's evidence of GI translocation, in which case a longer duration may be necessary. If the gastrointestinal barrier is disrupted, it's possible that an enteric organism can enter the blood stream and lead to septicemia.
Although antibiotics are important for recovery, supportive care is key to a successful outcome, too. “Most people believe that it's antibiotics that save the foal, but the reality is that supportive care is important, too,” he says. “This is an organism that will invade the small intestine, but eventually it will be eliminated.”
He also emphasizes the importance of rest and an easily digestible diet. If the animal is depressed and severely hypoproteinemic, colloids are recommended and can be found in plasma.

The foal pictured on page 1, three months later, fully recovered after therapy.
Prevention
Similar to monitoring foals for Rhodococcus equi, performing routine physical evaluations and blood work on a regular basis can prove helpful, especially when looking at trends of serum protein concentration. When infected with EPE, foals will become hypoproteinemic even before they develop clinical disease. Pusterla points out that it's also necessary to test for antibody levels and see if foals have been exposed.
For those who do not want to do careful monitoring, vaccination is recommended. There is a swine vaccine for L. intracellularis that has been shown to be safe and prevent disease in experimentally infected foals.
References
1. Pusterla N, Gebhart CJ. Equine proliferative enteropathy caused by Lawsonia intracellaris.Equine Vet Educ 2009;21(8):415.
2. Pusterla N, Gebhart CJ. Equine proliferative enteropathy–a review of recent developments. Equine Vet J 2013;45(4):403.
3. Pusterla N, Sanchez-McGallon, Guzman F, et al. Transmission of Lawsonia intracellularis to weanling foals using faeces from experimentally infected rabbits. J Equine Vet Sci 2012;32(10):S39.
4. Frazier ML. Lawsonia intracellularis infection in horses: 2005-2007. J Vet Intern Med 2008;22:1243.
Ed Kane, PhD, is a researcher and consultant in animal nutrition. He is an author and editor on nutrition, physiology and veterinary medicine with a background in horses, pets and livestock. Kane is based in Seattle.