Extramedullary and solitary osseous plasmacytomas in dogs and cats
Another important form of neoplastic plasma cells are plasmacytomas, which arise from soft tissue, where they are known as extramedullary plasmacytomas, or from bone, where they are known as solitary osseous plasmacytomas.
In the previous article, we focused on a diffuse type of plasma cell tumor—multiple myeloma. Another important form of neoplastic plasma cells are plasmacytomas, which arise from soft tissue, where they are known as extramedullary plasmacytomas, or from bone, where they are known as solitary osseous plasmacytomas.
SIGNALMENT AND PATHOLOGY
EMPs
Extramedullary plasmacytomas (EMPs) comprise about 2.5% of all neoplasms in dogs and occur most commonly in middle-aged to older dogs (mean 8 to 10 years).1-6 Overrepresented breeds include cocker spaniels, certain terrier breeds (West Highland white, Yorkshire, and Airedale terriers), boxers, and golden retrievers.1-6
In a large study evaluating 751 canine EMPs, the most frequent sites of origin were the skin (86% of plasmacytomas), mucous membranes of the oral cavity and lips (9%), and rectum and colon (4%). Other sites, including the stomach, small intestine, spleen, genitalia, and eye, represented 1% of plasmacytomas.3 Other reports suggest that oral plasmacytomas are more common and that about one-fourth of EMPs in dogs are within the oral cavity.4-6 Frequently reported locations for cutaneous tumors include the trunk, limbs, and head, with the ear being the most common site on the head.1,4,6 The true incidence of EMPs may be underestimated because of previous classification as reticulum cell sarcoma, neuroendocrine tumor, poorly differentiated round cell tumor, or cutaneous lymphoma.6,7
The incidence of EMP in cats is low, and few case reports exist. Affected cats are usually older, with a mean age of 8.5 years. Skin is the most common site affected, but other reported sites include the oral cavity, gastrointestinal tract, retroperitoneum, brain, and orbit.8-10
Antigenic stimulation, such as that resulting from gingivitis and periodontal disease, has been speculated to be a factor in the development of oral EMP. This hypothesis is supported by the identification of mature T cells and dendritic cells within oral EMP, suggesting both neoplastic and inflammatory components.2
SOPs
Solitary osseous plasmacytoma (SOP) is rarely reported in dogs and cats, and most cases progress to multiple myeloma months to years after local tumor development. Reported sites include the vertebrae, zygomatic arch, and rib.11-13 Anecdotally, we have also seen cases of SOP in which long bones are affected. In people, SOPs are most often found in the axial skeleton, usually the vertebrae and skull.14
CLINICAL SIGNS
EMPs
Clinical signs, other than the visible tumor mass or oral bleeding, are uncommon in most dogs with localized mucocutaneous EMP.2,6 Tenesmus, rectal prolapse, hematochezia, and rectal bleeding are the most frequent clinical signs in dogs with colorectal plasmacytomas.3,6 In case reports, EMPs of the larynx and trachea are associated with dysphonia and dyspnea, respectively, and intracranial EMP is associated with central nervous system signs.7,15,16 Globulin-secreting EMPs have been reported, and in these rare cases, clinical signs consistent with hyperviscosity syndrome may be present.4,17,18
The most common appearance of mucocutaneous EMP is a smooth, raised, red nodule, often 1 to 2 cm in diameter (Figure 1). Infrequently, dogs may develop polypoid, ulcerated, or multiple tumors.4,6 Reports of cutaneous tumors occurring with multiple myeloma are rare.19
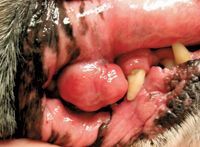
Figure 1. An oral EMP discovered on physical examination in a boxer. (Photograph courtesy of Dr. Laura Garrett.)
SOPs
Patients with SOP may present with bone pain, lameness, pathologic fracture, or neurologic deficits, depending on the site affected. Paresis or paralysis may be present with vertebral lesions and spinal cord compression.11,14
DIAGNOSIS AND STAGING
EMP is most often diagnosed by using cytology or immunohistochemistry. Histologic findings of EMP reveal that these tumors are nonencapsulated and locally destructive.5 A histologic classification system has been developed that may be helpful in diagnosing plasmacytomas, but there is no correlation among cell type, biologic behavior, and prognosis.1,5 Localized amyloid deposition may be detected in some tumors, and immunohistochemisty may demonstrate immunoglobulin light chain (lambda or kappa) expression. Lambda light chain monoclonality is observed in most dogs with EMP.1,5,20 The immunohistochemical marker multiple myeloma 1/interferon regulatory factor 4 (MUM1/IRF-4) may also assist in diagnosis.21
It is important to consider thorough staging in dogs and cats before pursuing therapy for EMP and SOP to rule out multiple myeloma and ensure that there is no evidence of systemic illness. Staging is especially important with plasmacytomas that tend to be associated with more aggressive disease, such as SOP and noncutaneous, non-oral EMP. Staging tests include skeletal radiography, bone marrow aspiration, and serum protein electrophoresis. In cases of oral and cutaneous EMP, which tend to be benign, staging is less important, and a minimum database and local lymph node aspiration are likely to be adequate before pursuing treatment. Imaging studies of oral EMP, such as high-detail dental radiography or computed tomography, may improve assessment of tumor infiltration and optimize surgical planning (Figure 2).2
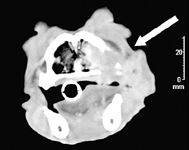
Figure 2. A transverse computed tomography image of the oral cavity in a dog with a maxillary EMP. The mass is locally invasive and destructive to the underlying bone (arrow). (Image courtesy of Dr. Laura Garrett.)
TREATMENT AND PROGNOSIS
EMPs
Unlike multiple myeloma, EMP generally carries a favorable prognosis. These tumors tend to be locally invasive but have a low metastatic rate, and complete surgical removal is often curative. In compilations of reports of canine cutaneous and mucocutaneous EMPs, surgical excision was curative in about 90% to 95% of cases. Local recurrence rates of 5% to 8% were encountered most often with microscopically incomplete resection, and distant metastasis was present in < 4% of cases.1,4,6 In cases of incomplete excision, additional surgery, adjunctive radiation therapy, or systemic chemotherapy may extend the disease-free interval.2,4 Multiple cutaneous EMP that is aggressive in behavior has been reported in dogs.22 Additionally, progression of EMP to multiple myeloma in cats and dogs has been reported.23,24
Noncutaneous, non-oral EMPs are reported to have a more aggressive behavior in dogs (Figure 3). However, dogs treated with surgery alone or a combination of surgery and adjuvant therapy can have extended survival times.3,18,25 In a report of nine dogs treated surgically for colorectal plasmacytomas, the median survival time was 15 months. In two cases in which surgical margins were incomplete or close, the tumor recurred locally five and eight months after resection (Figure 4).3 Adjuvant chemotherapy is recommended if metastatic disease is detected or if incomplete surgical margins are obtained. Systemic chemotherapy has been used after the excision of a globulin-secreting hepatic plasma cell tumor in a dog.18
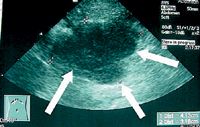
Figure 3. An ultrasonogram of a metastatic abdominal lymph node in a dog with an intestinal EMP. (Image courtesy of Dr. Laura Garrett.)
Few reports exist regarding cats with EMP. In a study of cats with myeloma-related disorders, cats with cutaneous plasmacytomas that underwent excision of the masses had a prolonged median survival of 663 days vs. 93 days in cats that received corticosteroids alone or no treatment. However, case reports have also suggested that some cutaneous EMPs may have a more aggressive behavior in cats.8
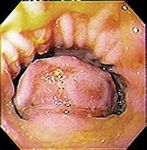
Figure 4. A colonoscopic image showing a colonic EMP in a dog. (Photograph courtesy of Dr. Laura Garrett.)
SOPs
Most cases of SOP reported in dogs ultimately progress to multiple myeloma. However, these patients may have long disease-free intervals before disease progression.11,12 The optimal treatment for most patients with SOP is radiation therapy administered at a total dose of 40 to 50 Gy given in daily fractions for three to four weeks.11,14,26 Depending on the tumor's location, surgical excision may also be an option.12 Systemic chemotherapy can be considered at the time of local treatment of SOP but is controversial in the absence of systemic involvement.22
SUMMARY
In general, EMPs often have a benign clinical course in dogs and cats and may be cured with complete surgical excision. Noncutaneous, non-oral EMP may have a more aggressive behavior; however, dogs treated with surgery alone or a combination of surgery and adjuvant systemic chemotherapy can have extended survival times. Most cases of SOP ultimately progress to multiple myeloma. Thorough staging before pursuing therapy for noncutaneous, non-oral EMP and SOP is important to rule out systemic disease.
Rachel Sternberg, DVM
Jackie Wypig, DVM DACVIM (oncology)
Department of Veterinary Clinical Medicine
College of Veterinary Medicine
University of Illinois
Urbana, IL 61802
Anne M. Barger, DVM, DACVP
Department of Pathology
College of Veterinary Medicine
University of Illinois
Urbana, IL 61802
REFERENCES
1. Cangul IT, Wijnen M, Van Garderen E, et al. Clinico-pathological aspects of canine cutaneous and mucocutaneous plasmacytomas. J Vet Med A Physiol Pathol Clin Med 2002;49(6):307-312.
2. Wright ZM, Rogers KS, Mansell J. Survival data for canine oral extramedullary plasmacytomas: a retrospective analysis (1996-2006). J Am Anim Hosp Assoc 2008;44(2):75-81.
3. Kupanoff PA, Popovitch CA, Goldschmidt MH. Colorectal plasmacytomas: a retrospective study of nine dogs. J Am Anim Hosp Assoc 2006;42(1):37-43.
4. Clark GN, Berg H, Engler SJ, et al. Extramedullary plasmacytomas in dogs: results of surgical excision in 131 cases. J Am Anim Hosp Assoc 1992;28:105-111.
5. Platz SJ, Breuer W, Pfleghaar S, et al. Prognostic value of histopathological grading in canine extramedullary plasmacytomas. Vet Pathol 1999;36(1):23-27.
6. Rakich PM, Latimer KS, Weiss R, et al. Mucocutaneous plasmacytomas in dogs: 75 cases (1980-1987). J Am Vet Med Assoc 1989;194(6):803-810.
7. Weight AK, McCracken MD, Krahwinkel DJ. Extramedullary plasmacytoma in the canine trachea: case report and literature review. Compend Contin Educ Pract Vet 2001;23(2):143-152.
8. Mellor PJ, Haugland S, Murphy S, et al. Myeloma-related disorders in cats commonly present as extramedullary neoplasms in contrast to myeloma in human patients: 24 cases with clinical follow-up. J Vet Intern Med 2006;20(6):1376-1383.
9. Majzoub M, Breuer W, Platz SJ, et al. Histopathologic and immunophenotypic characterization of extramedullary plasmacytomas in nine cats. Vet Pathol 2003;40(3):249-253.
10. Greenberg MJ, Schatzberg SJ, deLahunta A, et al. Intracerebral plasma cell tumor in a cat: a case report and literature review. J Vet Intern Med 2004;18(4):581-585.
11. Rusbridge C, Wheeler SJ, Lamb CR, et al. Vertebral plasma cell tumors in 8 dogs. J Vet Intern Med 1999;13(2):126-133.
12. MacEwen EG, Patnaik AK, Hurvitz AI, et al. Nonsecretory multiple myeloma in two dogs. J Am Vet Med Assoc 1984;184(10):1283-1286.
13. Mellor PJ, Polton GA, Brearley M, et al. Solitary plasmacytoma of bone in two successfully treated cats. J Feline Med Surg 2007;9(1):72-77.
14. Mendenhall WM, Mendenhall CM, Mendenhall NP. Solitary plasmacytoma of bone and soft tissues. Am J Otolaryngol 2003;24(6):395-399.
15. Hayes AM, Gregory SP, Murphy S, et al. Solitary extramedullary plasmacytoma of the canine larynx. J Small Anim Pract 2007;48(5):288-291.
16. Sheppard BJ, Chrisman CL, Newell SM, et al. Primary encephalic plasma cell tumor in a dog. Vet Pathol 1997;34(6):621-627.
17. Jackson MW, Helfand SC, Smedes SL, et al. Primary IgG secreting plasma cell tumor in the gastrointestinal tract of a dog. J Am Vet Med Assoc 1994;204(3):404-406.
18. Aoki M, Kim T, Shimada T, et al. A primary hepatic plasma cell tumor in a dog. J Vet Med Sci 2004;66(4):445-447.
19. Mayer MN, Kerr ME, Grier CK, et al. Immunoglobulin A multiple myeloma with cutaneous involvement in a dog. Can Vet J 2008;49(7):694-702.
20. Platz SJ, Breuer W, Geisel O, et al. Identification of lambda light chain amyloid in eight canine and two feline extramedullary plasmacytomas. J Comp Pathol 1997;116(1):45-54.
21. Ramos-Vara JA, Miller MA, Valli VE. Immunohistochemical detection of multiple myeloma 1/interferon regulatory factor 4 (MUM1/IRF-4) in canine plasmacytoma: comparison with CD79a and CD20. Vet Pathol 2007;44(6):875-884.
22. Vail DM. Plasma cell neoplasms. In: Withrow SJ, Vail DM, eds. Withrow & MacEwen's small animal clinical oncology. St. Louis, Mo: Saunders Elsevier, 2007;769-784.
23. Radhakrishnan A, Risbon RE, Patel RT, et al. Progression of a solitary, malignant cutaneous plasma-cell tumour to multiple myeloma in a cat. Vet Comp Oncol 2004;2(1):36-42.
24. Lester SJ, Mesfin GM. A solitary plasmacytoma in a dog with progression to a disseminated myeloma. Can Vet J 1980;21(10):284-286.
25. Brunnert SR, Dee LA, Herron AJ, et al. Gastric extramedullary plasmacytoma in a dog. J Am Vet Med Assoc 1992;200(10):1501-1502.
26. Dimopoulos MA, Kiamouris C, Moulopoulos LA. Solitary plasmacytoma of bone and extramedullary plasmacytoma. Hematol Oncol Clin North Am 1999;13(6):1249-1257.