Dentistry A to Z: G is for genetics
Oral health diseases in dogs and cats can be hereditary
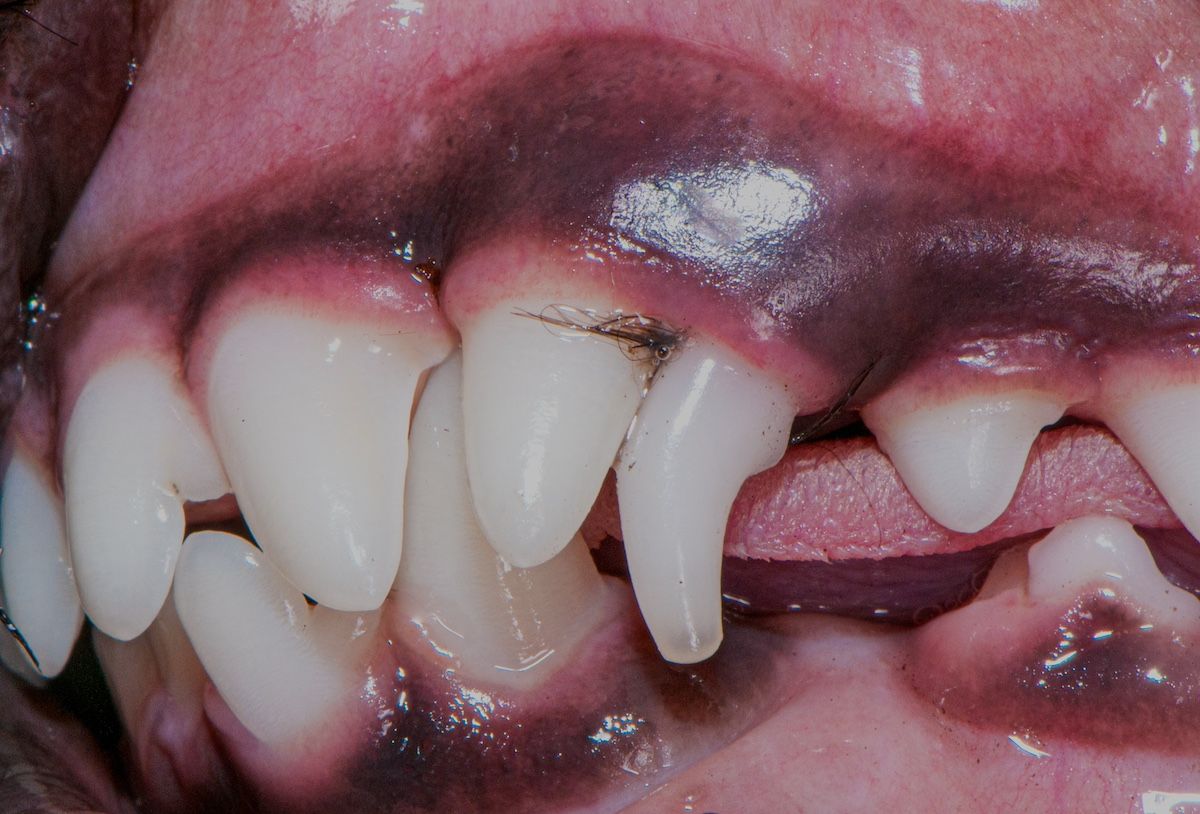
Figure 1a: Lance canine tooth in a Shetland sheepdog.

Oral health is a cornerstone of overall well-being. Although factors such as diet and hygiene influence dental health, genetics also play a significant role. For some oral diseases, the genetic basis has been confirmed, whereas a breed predisposition has been identified for others. For example, certain breeds with crowded teeth and skeletal malocclusion may have anatomical predispositions that facilitate bacterial accumulation.
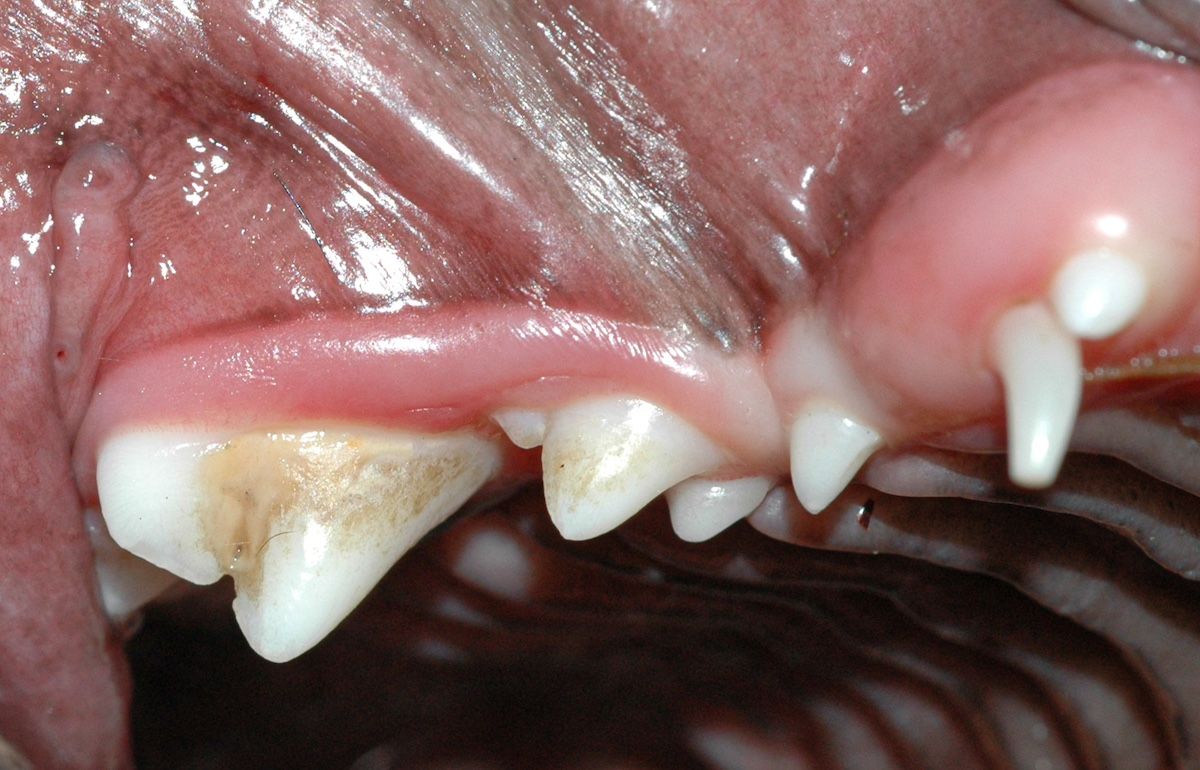
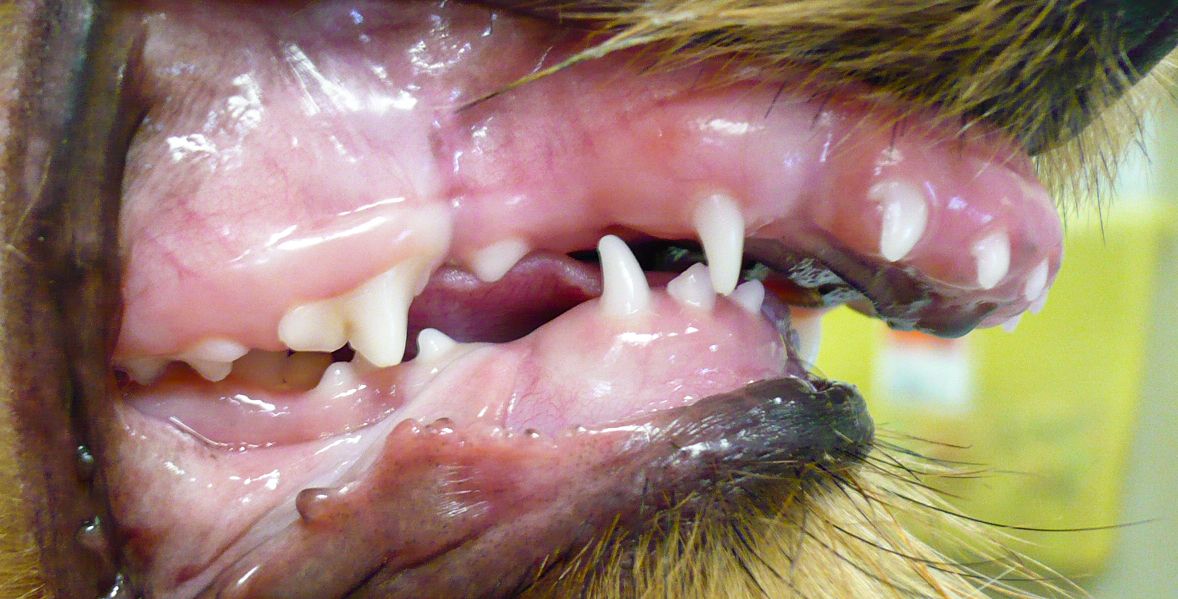
Figure 1b: Malpositioned second premolar and persistent primary canine tooth in a 7-month-old pug.
Clinical importance
Jerzy Gawor, DVM, PhD, DAVDC, DEVDC, worked with the Polish Small Animal Veterinary Association (PSAVA) to establish an evidence-based system to document the occurrence of hereditary oral and maxillofacial defects in purebred dog populations. The PSAVA Dental Working Group developed a list of 49 breeds that are prone to single or multiple oral/maxillofacial defects, referencing literature on each breed-specific condition and recommending diagnostic criteria, including clinical assessment, imaging, and laboratory tests (histopathology and genetics). Moreover, each defect was scored from 1 to 3 based on its negative impact on the animal’s health and vital functions, with 3 being the most deleterious.1
Breed predispositions
Charts created by the PSAVA Dental Working Group are available here. These charts demonstrate specific dog and cat breeds that are genetically associated with dental disease (Figure 1a).1
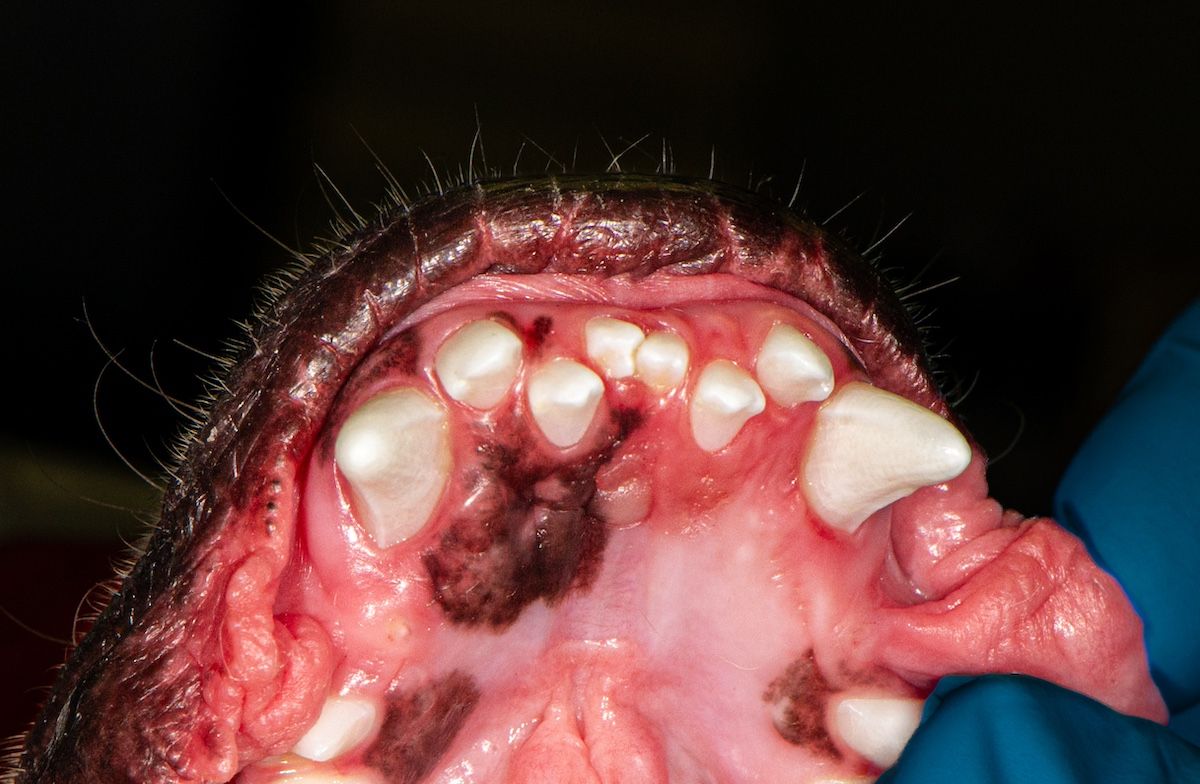
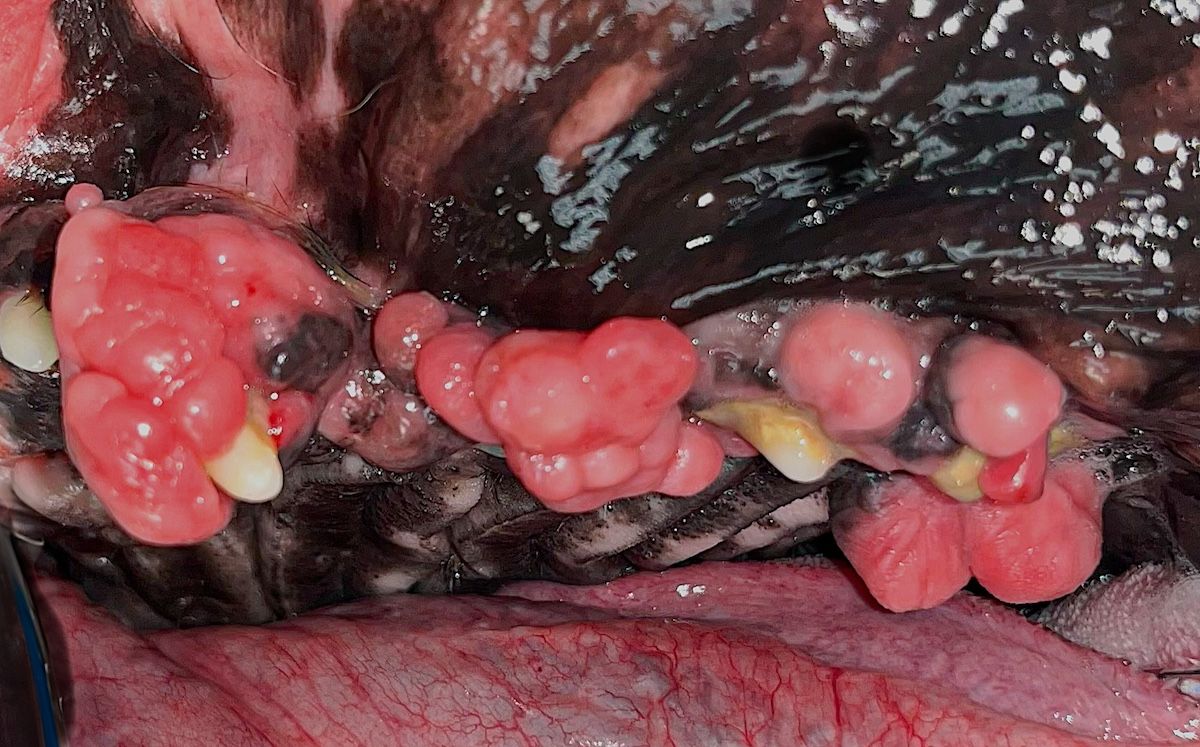
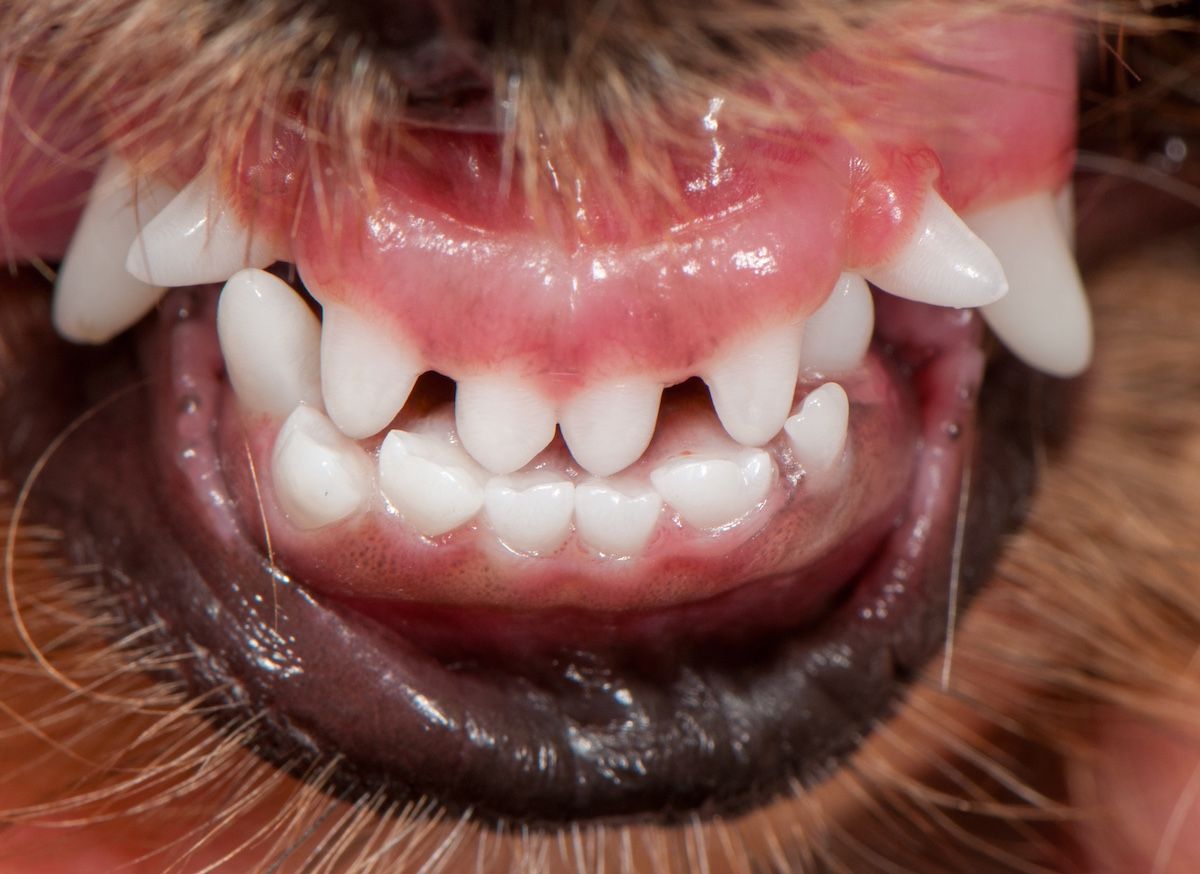
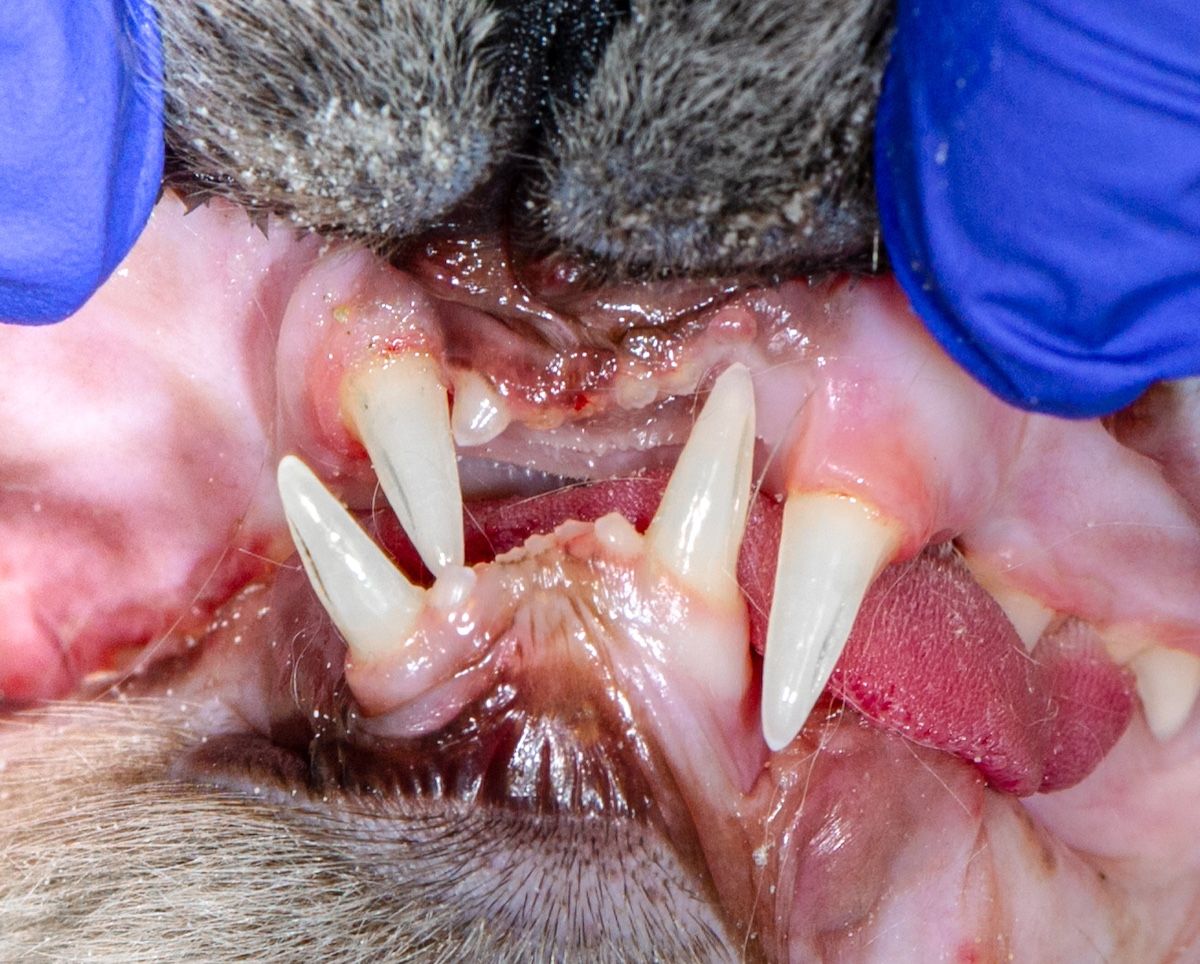
Brachycephalic breeds are predisposed to periodontal disease; these include flat-faced pugs, shih tzus, and bulldogs, as well as Persian, Scottish fold, and Himalayan cats. Their mouths do not have enough room to comfortably support 42 teeth for dogs and 30 teeth for cats (Figures 1b, 2a, 2b, and 2c).
Figure 2a: Crowded incisors in a Persian cat.
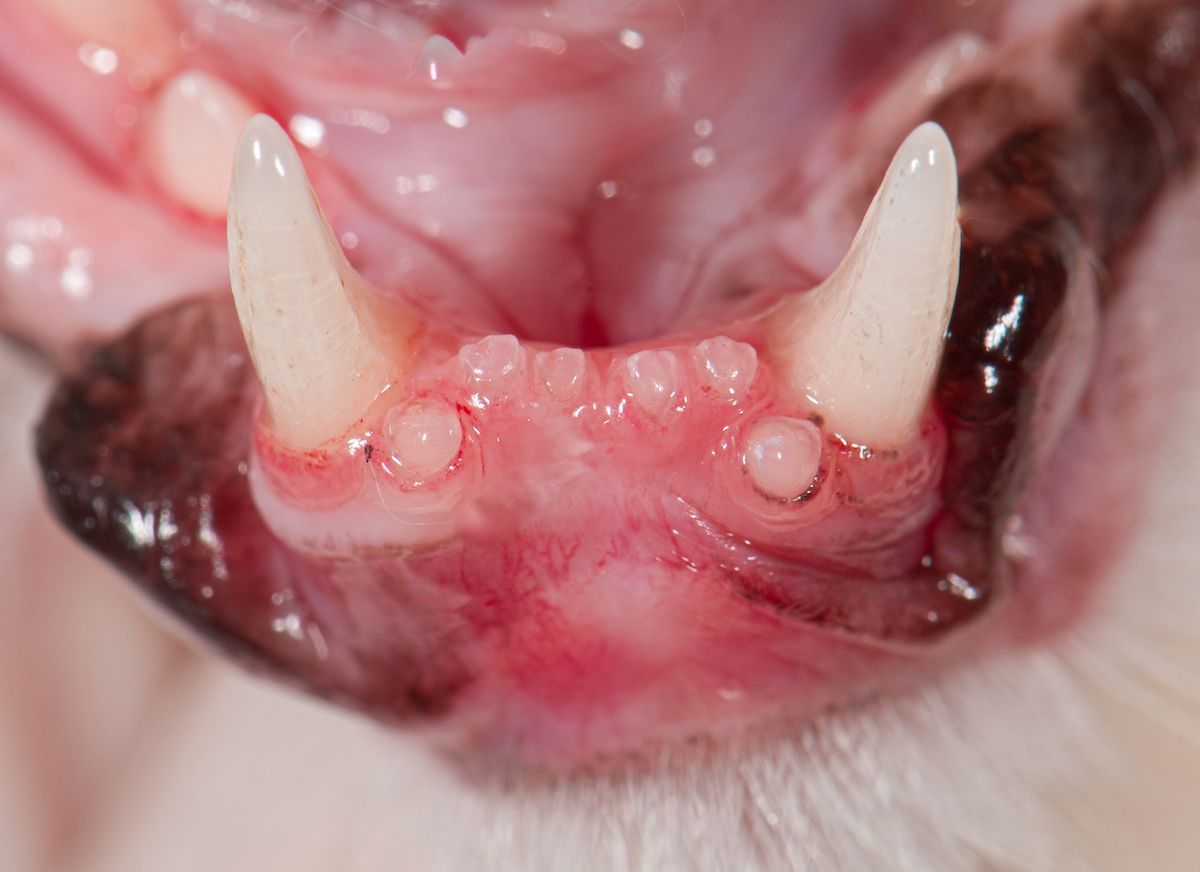
Figure 2b: Crowded incisors in an English bulldog.
The teeth of toy breeds, such as Yorkshire terriers, Chihuahuas, and Pomeranians, erupt closer together than larger breeds. This predisposes the toy breeds to increased plaque and tartar accumulation. A study published in the Veterinary Journal2 analyzed more than 3 million medical records (collected from 2010 to 2014) across 60 breeds of dogs visiting US Banfield locations. Statistical modeling of the data showed that extra small dog breeds were up to 5 times more likely to be diagnosed with periodontal disease than giant breeds (P < .0001). Additional risk factors for periodontal disease diagnosis included age, weight, and time since the professional dental scaling visit. Toy breeds are also genetically predisposed to persistent primary teeth (Figure 2d).
Oriental shorthair and Siamese cats may have a higher risk of tooth resorption. A Journal of Feline Medicine and Surgery study identified a potential genetic link to tooth resorption in Siamese cats, suggesting a breed predisposition.3
Figure 2c: Gingival enlargement in a boxer.
Genetic markers
Genetic markers include gene variations in inflammatory pathways, tooth development, and immune response.
Inflammatory mediators. Genes produce and regulate inflammatory molecules within the gingiva, including cytokines and chemokines. These play a role in the development and progression of periodontal disease. A study published in the Journal of Veterinary Dentistry investigated genetic variations of the IL-1 gene in dogs and found an association between specific polymorphisms and an increased risk of periodontal disease.4
Figure 2d: Persistent primary canine tooth in a Yorkshire terrier.
Although plaque and tartar accumulation from bacterial activity are the primary culprits triggering periodontal diseases, the genetic makeup of a dog or cat influences the immune response to these bacteria and the ensuing inflammatory cascade.
Tooth development genes. Variations in genes responsible for tooth formation and enamel development contribute to structural defects, increasing the risk of dental pathology.
Figure 3: Mandibular distoclusion mandibular mesioclusion (underbite).
Immune response genes. Genes that recognize and respond to bacterial pathogens, such as toll-like receptors (TLRs), are being studied for their potential role in periodontal disease susceptibility. A study published in the Journal of Veterinary Science explored genetic variations in the TLR9 gene in dogs and found an association with increased susceptibility to periodontal disease.5
Polygenic inheritance and modifier genes
Although specific genetic markers provide valuable insights, most oral pathologies in dogs and cats likely follow a polygenic inheritance pattern. Genes interact with environmental factors to determine susceptibility, each with a small effect. Because of the complex interplay, pinpointing the specific genes and their dominant or recessive nature is challenging. Moreover, modifier genes can further influence the expression and severity of a disease, even in the presence of a predisposing gene. Genes responsible for skeletal malocclusions, including mandibular distoclusion (overbite, overjet; Figure 3), mandibular mesioclusion (underbite; Figure 4), and maxillomandibular asymmetry (wry bite; Figure 5), are generally considered to be inherited dominantly. In contrast, individual tooth malposition is probably inherited recessively.
Figure 4: Mandibular mesioclusion and mandibular/maxillary asymmetry (wry bite).
The future of unraveling inheritance patterns
Advancements in veterinary genetics hold immense promise for understanding the inheritance patterns of oral pathologies in dogs and cats. Next-generation sequencing allows for detailed analysis of entire genomes, potentially revealing rare genetic mutations contributing to specific dental pathology.
Figure 5: Maxillomandibular asymmetry.
Genome-wide association studies are powerful tools for pinpointing specific gene variations associated with increased risk of dental disease. By analyzing the genomes of large animal cohorts, researchers can identify potential candidate genes for further investigation.
Gene-editing technologies, including clustered regularly interspaced short palindromic repeats (CRISPR)-Cas9 gene editing, have the potential to correct specific genetic mutations that increase susceptibility to dental disease. However, this technology has ethical considerations and needs extensive research before clinical applications.
Working with breeders to improve oral health
Breeders can reduce the prevalence of inherited dental issues by selecting breeding stock with good dental conformation and monitoring the incidence of oral pathology within their lines. By collaborating closely with breeders, veterinarians can help decrease the inherited nature of oral disease in purebred dogs and cats.
One of the first steps to accomplish this collaborative goal is identifying the genetic variations associated with oral health issues in different breeds. Genomic studies have already uncovered potential risk factors, such as variations in the IL-1 and TLR9 genes for periodontal disease susceptibility. However, more research is needed to establish robust genetic markers for various oral health conditions across breeds.6
Implementing genetic testing
Commercial genetic testing services, such as those offered by companies like Embark Vet and Paw Print Genetics, can provide valuable insights into an individual dog’s genetic predispositions. Once genetic markers are established, we can endorse genetic testing for breeding stock and potential breeding candidates. Breeders can avoid pairing 2 carriers by testing for known risk variants, reducing the likelihood of producing affected offspring. Additionally, genetic testing can help identify clear noncarriers, which can be prioritized for breeding to gradually reduce the prevalence of risk alleles within a breed population.
Takeaways
Although genetics play a significant role in dental health, proactive measures can help mitigate the risk of dental problems in predisposed breeds. Semiannual dental examinations, professional cleanings, and at-home dental care— including daily tooth wipes, brushing, and dental products accepted by the Veterinary Oral Health Council—are essential for all pets, especially those with genetic predispositions.
As research unveils the relationship between genes and oral health, we will move toward more personalized dental care strategies and improved overall patient well-being. The future of veterinary dentistry promises exciting advancements that will benefit pets for generations.
For recent updates to the Dentistry A to Z series:
- ABCs of dentistry: Airway, breathing, circulation
- E is for elegant extraction
- F is for fractured teeth
- Z is for zebras and other dental surprises
References
- Gawor J. Hereditary oral disorders in pedigree dogs. proposals for their evidence and assessment. European Journal of Companion Animal Practice. Accessed July 3, 2024. https://jukuri.luke.fi/bitstream/handle/10024/554104/Liite%202%20hammasongelmat.pdf;jsessionid=5206B9792FCCD394992FB8C25E881874?sequence=5
- Wallis C, Saito EK, Salt C, Holcombe LJ, Desforges NG. Association of periodontal disease with breed size, breed, weight, and age in pure-bred client-owned dogs in the United States. Vet J. 2021;275:105717. doi:10.1016/j.tvjl.2021.105717
- Whyte A, Tejedor MT, Whyte J, Monteagudo LV, Bonastre C. Blood parameters and feline tooth resorption: a retrospective case control study from a Spanish university hospital. Animals (Basel). 2021;11(7):2125. doi:10.3390/ani11072125
- Gonçalves-Anjo N, Requicha J, Teixeira A, Dias I, Viegas C, Bastos E. Genomic medicine in periodontal disease: old issue, new insights. J Vet Dent. 2022;39(4):314-322. doi:10.1177/08987564221109102
- Gonçalves-Anjo N, Leite-Pinheiro F, Ribeiro R, et al. Toll-like receptor 9 gene in periodontal disease - a promising biomarker. Gene. 2019;687:207-211. doi:10.1016/j.gene.2018.11.060
- Silva C, Requicha J, Dias I, Bastos E, Viegas C. Genomic medicine in canine periodontal disease: a systematic review. Animals (Basel). 2023;13(15):2463. doi:10.3390/ani13152463
