How Canine Brain Cancer Studies Could Help Humans, Too
Research in dogs is informing the design of cancer studies in people, particularly with respect to immunotherapy.
Gliomas are the most common primary intracranial tumors in humans, representing over 80% of malignant brain tum­­ors.1 Glioblastoma, the most aggressive and deadly of the gliomas, carries a median 2-year survival rate of less than 30%.1,2 In dogs, gliomas are the second most common type of primary brain tumor.3 As in people, dogs with this cancer have poor prognosis—the average survival time with symptomatic therapy is about 2 months.3
But researchers from the Virginia-Maryland College of Veterinary Medicine at Virginia Tech and University of Minnesota (UMN) College of Veterinary Medicine are hoping to improve those statistics by studying immunotherapies that may slow growth or even shrink gliomas in dogs and, ultimately, humans.

So far, 12 dogs have participated in the ongoing Molecular Combinatorial Therapy for Canine Malignant Gliomas trial.4 And additional funding has been granted by the National Institutes of Health (NIH) National Cancer Institute to start a new round of clinical trials this year.
The recently awarded $9.2 million 5-year NIH grant will fund 4 approaches to treating brain cancer—specifically glioblastoma—being applied by research teams at Virginia Tech and Wake Forest University.5 All the funded projects include cancer therapies that have been tested in dogs.
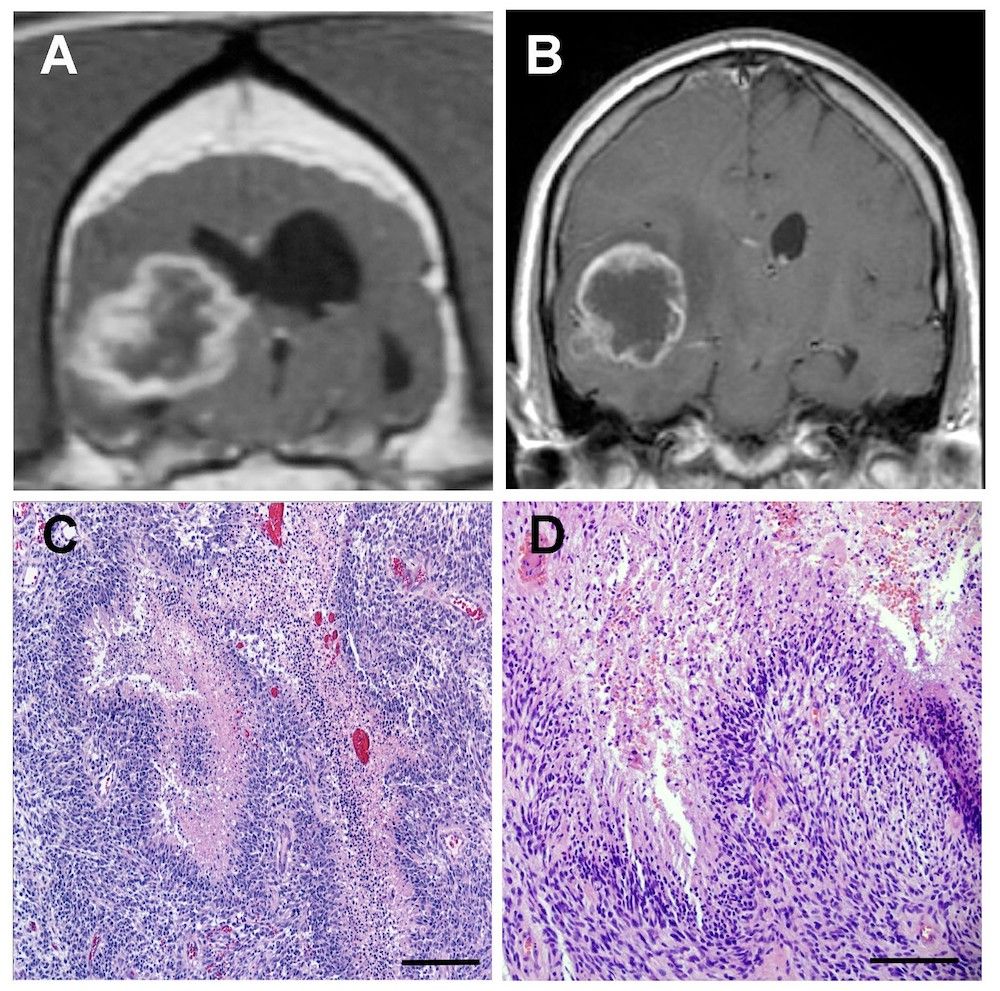
Work done in the Rossmeisl Laboratory at Virginia Tech has demonstrated that canine malignant gliomas share many clinical, radiologic, molecular, and pathologic features with their human counterparts. Shown here are the comparative phenotypic features of canine and human glioblastoma as demonstrated with magnetic resonance imaging and histopathology. Postcontrast, T1-weighted magnetic resonance images of glioblastoma in the brains of a dog (A) and human (B). Photomicrographs of canine (C) and human (D) glioblastoma biopsies (hematoxylin & eosin). Panel D courtesy of Dr. Constance Stanton, Wake Forest University School of Medicine, Department of Pathology. Other images from Virginia-Maryland College of Veterinary Medicine at Virginia Tech.
“The projects of the grant are novel in that they use newer, improved versions of the technologies or drugs based on results obtained in the lab as well as in clinical trials,” said John Rossmeisl, DVM, MS, professor of neurology at the Virginia-Maryland College of Veterinary Medicine at Virginia Tech and lead researcher of the canine clinical trials. “We are evaluating various combinations of these unique approaches as they may have synergistic and additive anticancer effects.”
The treatment being studied involves convection enhanced delivery (CED) of molecularly targeted cytotoxins to the patient’s brain tumor. With CED, cytoxins are delivered through specialized catheters inserted directly into the tumor and infused slowly over several hours.6
The goal for the study is to improve the lives of dogs with brain cancer and use the canine spontaneous brain tumor model to then develop novel therapeutics for humans with this type of brain cancer. Since the trial began, treated dogs have fared better than controls. Dr Rossmeisl said some of the tumors shrank, and some dogs lived a year longer than expected.
The research team hopes to enroll dogs into the clinical trial as quickly as possible once they have determined eligibility. To be eligible, dogs must meet the following criteria:
- Any age, breed, or sex
- Weight between 3 and 45 kg (6.6 to 99 lb)
- Clinical signs of mild to moderate neurologic dysfunction referable to the brain
- MRI evidence of a single telencephalic intra-axial mass lesion consistent with a glioma
- No clinical or other diagnostic evidence of other significant systemic disease
Dogs with uncontrollable seizures and those not expected to survive the 3-week study period based on clinical tumor progression are ineligible for the study.
According to Mindy Quigley, clinical trials coordinator at the Virginia-Maryland College of Veterinary Medicine, the current NIH study is likely to continue and

Jake Pratt, a 5-year-old shepherd mix, was all smiles upon discharge from the Veterinary Teaching Hospital at Virginia Tech in March 2015 after undergoing surgery to treat a glioblastoma. Jake is pictured with his care team and owners (left to right): fourth-year veterinary student Meliasa Robinson, Dr. Rossmeisl, Teresa and Todd Pratt of Knoxville, TN, and fourth-year veterinary student Katelyn Somers.
transition into the new study this year with the same enrollment criteria but a different version of the chemotherapy drug.
“The current version of the drug targets 2 cell surface proteins that are present in glioma cells but not in healthy brain tissue,” Quigley said. “The team has since identified 2 additional markers, and the new version of the drug will target those additional markers, for a total of 4. That gives the drug 4 proteins to seek out and ‘grab.’”
With this rare of a cancer, participant recruitment alone can take several years. It will likely be many years before the research team can understand whether this new chemotherapy drug is effective, and several more years before human clinical trials are approved.
Quigley said there will almost definitely need to be more rounds of clinical trials because there are many different types of brain tumors that present in myriad ways. Gliomas also have several subtypes.
The research has a long way to go, but Quigley hopes that this study will be successful. “We have strong reason to believe the new version of the drug will be even more effective in identifying and killing the tumor cells,” she said.

A new 5-year research project at the University of Minnesota (UMN) College of Veterinary Medicine will study canine high-grade glioma to help find ways to improve treatment for dogs and, ultimately, humans. The project has received a $2.7 million grant funded as part of the 21st Century Cures Act by the National Cancer Institute to give researchers more insight into glioblastoma to apply to human trials.
Dr. Pluhar with Chomper, a French bulldog that underwent surgical removal of an anaplastic (grade III) oligodendroglioma followed by vaccinations using a lysate of the dog’s own tumor cells, imiquimod, and the canine-specific CD200 peptide developed by Dr. Mike Olin. Chomper was humanely euthanized after the tumor recurred about 9 months post surgery.
The research team involved in the Canine Brain Tumor Clinical Trial Program has treated more than 200 dogs to date.7,8 They have been experimenting with various treatments to better understand what does and does not work.
Previously, the team created vaccines from each dog’s own tumor cells—called tumor lysate—that incited a specific immune response against tumor cells. They also experimented with adenoviral-mediated gene therapy that prolonged progression-free times and overall survival of the dogs with no adverse effects.
Although these treatments demonstrated beneficial effects for most canine patients, the tumors still recurred over time, usually by 7 or 8 months.

That’s when Michael Olin, PhD, assistant professor in the Department of Pediatric Hematology and Oncology at UMN, discovered that the tumors secrete a substance that acts as a checkpoint inhibitor blocking the body’s natural immune response, called CD200, and created a canine-specific peptide that can override this interaction and allow a normal immune response.
For this newly funded project, investigators hope to demonstrate how a combination of immunotherapy with this newly created CD200 peptide and vaccines or gene therapy will prove safe and highly effective in dogs with high-grade gliomas and extend tumor-free and overall survival times to well over a year.
In addition, the research team is striving to learn as much as it can about the type of immune cells that kill tumor cells and delay disease progression, and whether molecular changes in tumor cells occur in response to immunotherapy.
At least 30 dogs with confirmed spontaneous glioma are needed for the study. The eligibility criteria are similar to those for the Virginia-Maryland study. Although dogs with meningioma are no longer being recruited, the university is offering to treat dogs diagnosed with meningioma using surgery and vaccine-based immunotherapy, which can provide tumor control for years.
References:
- Ostrom QT, Bauchet L, Davis FG, et al. The epidemiology of glioma in adults: a “state of the science” review. Neuro Oncol. 2014;16(7):896-913.
- Glioblastoma and malignant astrocytoma. American Brain Tumor Association website. http://www.abta.org/secure/glioblastoma-brochure.pdf. Published 2017. Accessed January 4, 2018.
- Canine glioma surgery. Purdue University College of Veterinary Medicine website. https://vet.purdue.edu/pcop/cgs.php. Accessed January 4, 2018.
- Molecular combinatorial therapy for canine malignant gliomas. Virginia-Maryland College of Veterinary Medicine website. http://www.vetmed.vt.edu/clinical-trials/current-studies/molecular-combinatorial-therapy.asp. Accessed December 20, 2017.
- Grant puts research team on track to treat brain cancer. Virginia Tech News website. https://vtnews.vt.edu/articles/2017/10/univrel-cancergrant.html. Published November 3, 2017. Accessed December 20, 2017.
- Convection enhanced delivery. Virginia-Maryland College of Veterinary Medicine website. http://www.vetmed.vt.edu/clinical-trials/canine-glioma/convection-enhanced-delivery.asp. Accessed January 4, 2018.
- Canine brain tumor clinical trials program. University of Minnesota College of Veterinary Medicine website. https://www.vetmed.umn.edu/centers-programs/clinical-investigation-center/current-clinical-trials/canine-brain-tumor-clinical-trials-program. Accessed January 4, 2018.
- Canine brain tumor research may speed human trials. University of Minnesota College of Veterinary Medicine website. https://www.vetmed.umn.edu/news/canine-brain-tumor-research-may-speed-human-trials. Published December 11, 2017. Accessed January 4, 2018.
