Introduction to common chemotherapy agents (Proceedings)
Goals of chemotherapy: tumor control, prolonged survival, maintain quality of life.
Please be aware that these notes are not designed to be a complete reference. It is advisable to consult with an oncologist for current treatment recommendations prior to developing a therapeutic plan for your patient.
Goals of Chemotherapy - Tumor control, prolonged survival, maintain quality of life.
Chemotherapy Theory
Gompertzian Growth Kinetics
Model that describes how tumors grow. Initially a tumor grows at an exponential rate. As the tumor becomes larger, the growth fraction (percentage of cells that are actively dividing) decreases. A tumor is detectable (1 cm diameter) at about 109 cells. Since actively dividing cells are more sensitive to DNA damage and damage to cellular processes, chemotherapy is more effective in rapidly growing tumors. According to Gompertzian growth kinetics, microscopic or small tumors are more likely to be rapidly growing and therefore more sensitive to chemotherapy. Therefore we want to treat when the smallest amount of disease is present.
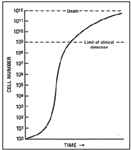
This model shows how tumors grow.
Goldie-Coldman Hypothesis
Cancer cells are genetically unstable. This means they accumulate additional spontaneous mutations as they proliferate. As a tumor grows, these mutations can make the cells resistant to chemotherapy, even without exposure to chemotherapy. Consequently, larger tumors are more likely to contain resistant tumor cells so we should use multiple effective drugs when the smallest about of disease is present to kill cells before resistance develops.
Cell Kill Hypothesis
Each dose of chemotherapy kills a constant fraction of the tumor cells. The number of cells killed is related to the dose given. The surviving cells proliferate between treatments. This hypothesis suggests that it is impossible for chemotherapy to eradicate all tumor cells and that we should treat with the highest tolerated doses (that are safe) given as frequently as is safe for the patient.
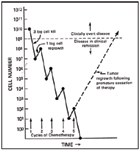
Cell kill hypothesis
Tumor Blood Supply
As a tumor grows, a blood supply must be grown from the host (angiogenesis). Tumor vessels are abnormal and there is a greater diffusion distance between vessels so larger tumors have decreased blood supply and decreased drug delivery. In addition, areas of hypoxia and decreased cell proliferation develop. Consequently, chemotherapy is not as effective for bulky tumors as for microscopic disease.
Resistance to Chemotherapy
There are many mechanisms of resistance to chemotherapy. Multi-drug resistance (MDR) is mediated by P-glycoprotein (P-170), a drug-efflux pump that removes foreign substances from the cell. This pump is normally expressed in fetal tissue, liver, lung, renal tubular cells, pancreas, intestine, brain, blood, and bone marrow. Cancer cells can up-regulate expression of P glycoprotein. Exposure to one drug affected by multi-drug resistance can lead to resistance to other drugs in this group. It is also important to note that some dogs can have mutations in the MDR-1 gene which codes normal P-glycoprotein, resulting in increased toxicity. These mutations have been documented primarily in herding and sighthound breeds including Collie, Australian Shepherd, English Shepherd, McNab, Old English sheepdog, Shetland sheepdog, German shepherd, Border collie, Longhaired whippet, and Silken windhound. Drugs affected by MDR include prednisone, doxorubicin, vincristine, mitoxantrone, vinblastine, actinomycin-D, taxanes, and other non-chemotherapy drugs.
Introduction to Commonly Used Chemotherapy Drugs
**Before using any chemotherapy agent, you are responsible for reviewing use, dose, and toxicity. Over time dose recommendations may change and new toxicities may be identified. In addition, you are responsible for the safety of yourself, your co-workers, patients, and the owners of your patients.**
Vincristine, Vinblastine, Vinorelbine – Antitubulin Agents, Plant Alkyloids
1. Brand Name: vincristine = Oncovin, Vincasar, vinblastine = Velban, vinorelbine = Navelbine
2. Mechanism of Action: Bind tubulin, causing mitotic spindle disassembly, metaphase arrest, cell death.
3. Administration: IV only, given as bolus.
4. Metabolism: Hepatic metabolism and biliary excretion. Dose reductions or not used if hepatic dysfunction. Small amount excreted in the urine.
5. Associated Toxicity
a. Extravasation injury – if occurs, aspirate back as much of drug as possible before removing catheter. Then inject 1 mL of hyaluronidase 150 μg/mL for each ml of extravasated drug and apply warm compresses for 10-15 minutes every 6 hours for 72 hours. Treatment with antiinflammatories (topical) and antibiotics may be helpful.
b. Gastrointestinal toxicity – delayed gi toxicity is possible. Paralytic ileus and constipation may be seen with vincristine. Hospitalization and CRI of metoclopramide may be necessary.
c. Myelosuppression – is possible, but uncommon with these drugs. Risk is higher with vinblastine than with vincristine. When vincristine is used in combination with L-asparaginase or cyclophosphamide, increased risk of myelosuppression.
d. Neuropathy – peripheral neuropathies are possible with vincristine, but rare in veterinary patients. Vincristine should be discontinued if this occurs. Vinblastine and vinorelbine less neurotoxic.
6. Uses: Vincristine - Lymphoma, lymphoid leukemias, transmissible venereal tumors, ITP. Vinblastine – Primarily for canine mast cell tumors, being investigated for feline mast cell tumors. Substituted for vincristine if ileus or neuropathy occurs. Vinorelbine – Being investigated for canine primary lung tumors.
L-Asparaginase – Enzyme
1. Brand Name: Elspar
2. Mechanism of Action: Degrades L-asparagine, an essential amino acid for lymphoid cancer cells, inhibiting protein synthesis.
3. Administration: Subcutaneous injection. Do not give IV or IP because of increased risk of anaphylaxis.
4. Metabolism: Mainly consumed in the reaction, but some excretion in urine and feces.
5. Associated Toxicity
a. Allergic reaction, anaphylaxis – With repeat exposures to this drug, the patient can have an allergic reaction to the foreign protein. Pre-treatment with diphenhydramine 2 mg/kg IM is recommended after the first dose of this drug. Monitor closely after administration for reaction. Discontinue if allergic reaction.
6. Uses Lymphoma, acute lymphoblastic leukemia
Cyclophosphamide – Alkylating Agent
1. Brand Name: Cytoxan
2. Mechanism of Action: Attaches alkyl group to DNA. Results in DNA damage, inhibits synthesis of DNA, RNA, and proteins. Results in apoptosis (cell death). Not cell-cycle phase specific.
3. Administration: IV bolus or PO (supplied as 25 mg or 50 mg tablets, do not split because the drug is not evenly distributed throughout the tablet and aerosolization is dangerous).
4. Metabolism: Activated by hepatic metabolism. Mainly excreted in urine.
5. Associated Toxicity
a. Myelosuppression – check CBC 1 week after treatment.
b. Gastrointestinal – both acute and delayed toxicity are possible, but uncommon.
c. Hemorrhagic Cystitis – cyclophosphamide is metabolized to an inactive metabolite (acrolein) which is directly irritating to the bladder epithelium and can cause a sterile hemorrhagic cystitis. This toxicity is rare in cats. To prevent hemorrhagic cystitis, cyclophosphamide should be given in the morning, with furosemide (2.2 mg/kg at same time as cyclophosphamide is given) to induce PU/PD. Water should be available at all times and the dog should be allowed to urinate frequently for the 24 hours after administration of this drug. Avoid giving this drug if the patient has active cystitis. If hemorrhagic cystitis develops, the dog should be checked for infection and treated if present. Hemorrhagic cystitis is generally self-limiting but symptomatic treatment with anti-inflammatory agents and oxybutinin may be palliative. If a dog develops hemorrhagic cystitis, he/she should not receive cyclophosphamide again. Chlorambucil (has a different dose than cyclophosphamide –see the protocol you are using) is an alkylating agent that may be substituted for cyclophosphamide.
6. Uses Lymphoma, lymphoid leukemias, carcinomas, sarcomas.
Doxorubicin – Antitumor Antibiotic
1. Brand Name: Adriamycin
2. Mechanisms of Action
a. Activates cell signaling pathways signaling the cell to die (apoptosis).
b. Inhibits topoisomerase II (enzyme responsible for separating and reattaching DNA strands).
c. Intercalates into DNA, causing damage and interfering with production of DNA, RNA, and proteins.
d. Generates free radicals which damage DNA and cancer cells.
3. Administration: IV, through a perfectly placed catheter only. Administered IV as a slow infusion at 1 mg/min. Heparin precipitates this drug. Constant observation of the injection site is essential. Cats and small dogs are overdosed with body surface area dosing of this drug. For dogs >1 m2, dose is 30 mg/m2. For dogs < 1 m2, dose is 1 mg/kg. For cats, dose is 20 mg/m2, if weight less than 2.5 kg, dose is 1 mg/kg.
4. Metabolism: Hepatic metabolism. Primarily biliary excretion, small amount in urine.
5. Associated Toxicity
During Administration
a. Severe extravasation injury if leaks out of vein. If occurs, aspirate as much of drug back as possible before removing catheter. Then apply cold compresses for 15 minutes at least every 6 hours for the next 72 hours. If large amount has been extravasated, surgical resection might be a consideration. Do not infuse area. *New experimental and anecdotal evidence suggests that IV treatment with dexrazoxane (Zinecard) given within 3 hours of extravasation may help. Protocol is in Plumb Formulary. Treatment with topical 90% DMSO every 8 hours may be also helpful. Vein cannot be used again for chemotherapy.
b. Allergic-type reaction – doxorubicin can stimulate mast cells to release histamine, resulting in facial swelling, pruritis, urticaria, gi signs (more likely in dogs), dyspnea (more likely in cats), hypotension, and shock. Slow administration will help prevent this toxicity.
c. Arrhythmias – associated with mast cell release of histamine and catecholamines that irritate myocardium. Risk is reduced with slow administration.
d. Acute gi toxicity – possible, but uncommon. Risk reduced with slow administration.
Delayed Toxicity
a. Myelosuppression – need CBC 1 week after treatment.
b. Gastrointestinal toxicity – vomiting, diarrhea, lethargy, and loss of appetite 2-5 days after treatment. Doxorubicin has a unique toxicity of hemorrhagic colitis in dogs that can be life-threatening. The most common toxicity of this drug in cats is loss of appetite.
c. Cardiac toxicity – free radical injury to myocardium (low levels of catalase) resulting in arrhythmias, irreversible DCM, and congestive heart failure in dogs. Usually seen at cumulative doses of 180-240 mg/m2. In cats, histologic changes, but no clinical cardiac toxicity. Breeds at risk for DCM and dogs with existing cardiac abnormalities (murmur, arrhythmia, cardiomegaly) should have an echocardiogram before administration of this drug. Dose is usually capped at 150 mg/m2 for dogs. All dogs should routinely have an echocardiogram after 150 mg/m2 cumulative dose if considering further doses. Dexrazoxane (Zinecard) has been used with doxorubicin in humans to prevent cardiac toxicity. Not used routinely in dogs at this time. For dogs unable to receive doxorubicin because of cardiac toxicity, mitoxantrone or actinomycin-D (at their appropriate doses) may be substituted.
d. Nephrotoxicity – poorly characterized toxicity in cats, possibly dogs. Believed to be cumulative toxicity.
6. Uses: Lymphoma, ALL, hemangiosarcoma, osteosarcoma, other sarcomas, carcinomas
Carboplatin – Platinum Drugs
This drug is now available in generic formulation and has largely replaced the use of cisplatin because of easier administration and more tolerable toxicity profile.
1. Brand Name: Paraplatin
2. Mechanism of Action: Aquated to active form. Binds to DNA, RNA, and proteins, resulting in damage and interference with the synthesis of DNA, RNA, and proteins. Cell cycle phase non-specific.
3. Metabolism: Renal excretion. Use at reduced dose or not at all in animals with decreased renal function because severe myelosuppresion is possible.
4. Administration: Given intravenously over 5–10 minutes. Can be used in cats. Cats and small dogs are overdosed with body surface area dosing of this drug and therefore are treated with lower doses than large dogs. Cats:150-210 mg/m2, 10 mg/kg Dogs: small dogs:200-250 mg/m2 large dogs:250-300mg/m2
5. Toxicity
a. Myelosuppression – carboplatin is very myelosuppressive. The nadir can occur 1, 2, or 3 weeks after treatment (but usually between days 11-14 for dogs and days 14-21 for cats). CBCs should be performed weekly after the first treatment. Cats can have prolonged myelosuppression.
b. Gastrointestinal toxicity – uncommon. Can be administered without prophylactic antiemetic therapy.
c. Nephrotoxicity – rare, but has been reported.
6. Uses: Carcinomas, melanoma, osteosarcoma, other sarcomas.
CCNU (Lomustine) – Alkylating Agent
1. Brand Name: CeeNu
2. Mechanism of Action: Attaches alkyl group to DNA. Results in DNA damage, inhibits synthesis of DNA, RNA, and proteins. Not cell-cycle phase specific.
3. Administration: PO. Comes as 10 mg, 40 mg, or 100 mg capsules. Do not open capsules. Can be reformulated to appropriate sizes. Cats and small dogs require lower doses of this drug. For large dogs, 70 mg/m2 every 3-4 weeks. For cats, 50 mg/m2 or 10 mg/cat every 4-6 weeks is recommended.
4. Metabolism: Mainly excreted in urine. Some hepatic metabolism.
5. Associated Toxicity
a. Myelosuppression – is very common with this drug. Need CBC 1 week after treatment. Can see severe neutropenia, especially at the higher end of the dose range. Neutrophil counts may drop to <500 cells/μL. Consider prophylactic antibiotic therapy the first time a dog receives this drug, starting 4 days after administration and continuing to day 7 if there is not severe neutropenia, or day 14 if there is severe neutropenia. Sepsis is not common with this drug.
b. Gastrointestinal – rare, occasionally see diarrhea.
c. Cumulative delayed myelosuppression – severe bone marrow hypoplasia is possible. Generally manifests first as thrombocytopenia. Can progress even after discontinuing drug and may never return to normal. Check CBC before each dose. CCNU should not be administered if the platelet count is <200,000 cells/μL. This toxicity may occur more quickly at a 3-week dosing interval than at a 4-week dosing interval.
d. Hepatotoxicity – non-specific liver injury. Check chemistry profile every other dose. Initially elevated liver enzymes, then progresses to clinical liver disease, and possibly death. CCNU should not be used if liver function is abnormal and should be discontinued if liver enzymes are increasing.
e. Renal toxicity – possible, not well described.
6. Uses: Resistant lymphoma, mast cell tumors, use in cats is newer.
Piroxicam – Oxicam Non-Steroidal Anti-Inflammatory Drug (NSAID)
1. Brand Name: Feldene
2. Mechanism of Action: COX 2 inhibitor – results in decreased tumor-associated inflammation, b. Anti-tumor effect is poorly understood. Possibly stimulates apoptosis (cell death), possibly anti-angiogenic (blocks tumor growth of blood vessels from the host), and possibly immunomodulatory.
3. Administration: PO, with food - 10 mg and 20 mg capsules. Narrow therapeutic index - must be reformulated for most dogs to dose at 0.3 mg/kg/day. Not used much in cats. Has been dosed at 0.3 mg/kg/48 hr in cats, but use of this drug in cats is not well worked out (has not been effective and toxicity has been noted).
4. Metabolism: Metabolized in the liver and excreted primarily in the urine.
5. Associated Toxicity
a. Gastrointestinal ulceration – more likely to cause gi toxicity than some more commonly used veterinary NSAIDs. Dose carefully. Some dogs will not tolerate every day dosing and should be dosed every other day. Monitor for gi signs, dark stool, and check PCV/TS and chemistry profile after first 1-2 weeks on this drug, then every 1-2 months. The prostaglandin E1 analogue misoprostol may help prevent gi toxicity but most dogs do not need misoprostol. Misoprostol causes abortions in dogs and women. You are responsible for warning owners and should not send this drug home to a household with a pregnant woman. Also, misoprostol itself can cause gi signs. Do not give piroxicam with prednisone or other NSAIDs; this will cause gi ulcers.
b. Renal papillary necrosis – rare, but possible. Do not give this drug with cisplatin, too nephrotoxic in combination. Check chemistry profile after first 1-2 weeks on this drug, then every 1-2 months.
c. Inhibition of platelet function – we do not see this clinically.
6. Uses: Primarily carcinomas. Best evaluated for transitional cell carcinoma and oral squamous cell carcinoma in dogs. Responses also seen with pulmonary carcinoma, nasal carcinoma, mammary carcinoma, inflammatory mammary carcinomas, and prostatic carcinoma. Safer NSAIDs are now being investigated for anticancer efficacy.