Managing infectious equine neurological disease (Proceedings)
Neurological disease represents 0.3% (affecting between 0.2 and 0.5% of horses depending on age) of all health problems identified by owners in the latest 2005 Equine National Animal Health and Monitoring Study (NAHMS).14 Likely this is much higher given losses in young horses due to non-infectious neurological causes, in all ages of horses from underreporting of encephalitis, and misdiagnoses of these diseases as lameness and trauma.
Neurological disease represents 0.3% (affecting between 0.2 and 0.5% of horses depending on age) of all health problems identified by owners in the latest 2005 Equine National Animal Health and Monitoring Study (NAHMS).14 Likely this is much higher given losses in young horses due to non-infectious neurological causes, in all ages of horses from underreporting of encephalitis, and misdiagnoses of these diseases as lameness and trauma. The actual contribution of neurological impairment to the health of US equids overall is actually unknown due to poor diagnostic tools, loss of animals to post-mortem testing if destroyed, and reporting mechanisms that emphasize diseases only of human significance. In fact, while owners in the 2005 NAHMS study only identified 5.0% of the non-ambulatory horses as neurological, another 30.6% were due to a "lameness" problem which may have been incorrectly categorized. Neurological impairment in the horse, even more so than lameness or trauma, is more apt to foreshorten the animal's athletic career, result in permanent deficit, monetary investment by the owner for treatment, and ultimately destruction of the animal. Sixty-one percent of those horses were rendered unusable in the 1998 NAHMS data on EPM, either these horses relapsed, did not respond to treatment, were destroyed or sold due to the original diagnosis. If one estimates for 2010 that approximately 6M equids reside in the US and if one uses an overall estimate of 0.3% population incidence of neurological disease, at least 180,000 cases of some sort of neurological disease occur annually in horses. If a conservative 50% of those animals do not survive, then this represents a loss of 90,000 horses per year to neurological impairment. In direct value, utilizing the AHC data, we performed a survey during the WNV outbreak (unpublished data) and based on client reporting, the direct value of the horses averaged $1500/horse thus a loss of 90K horses represents 270M in livestock loss alone. We further estimated based on American Horse Council statistics that each horse represents $14K/year in combined direct and indirect benefits to the economy. Thus the loss due to equine neurological impairment just from mortality is conservatively 1.2B per year.
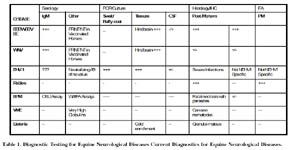
Table 1. Diagnostic Testing for Equine Neurological Diseases Current Diagnostics for Equine Neurological Diseases.
Antemortem testing for the cause of equine neurological disease is still more art than science. Causes of neurological diseases in the horse are infectious (RNA viruses, DNA virus, parasitic, bacterial and fungal) and noninfectious (cervical vertebral malformation, various congenital malformation, degenerative, toxic, and trauma).13 Given the substantial loss due to equine neurological disease, evidence-based identification of the causes of equine neurological diseases poses a constant challenge and this is estimated at 60% for a confirmed diagnosis (based on our database of over 2900 post mortem cases of neurological disease) while less than 30% are found to have had the etiology identified ante-mortem.14-16 Furthermore, the CNS is an immunologically privileged site which is difficult to access in the standing normal or neurologically impaired horse, thus "organ-specific" diagnosis is a challenge without invasive procedures. Although the singlemost valuable test one can obtain is a CSF analysis, many times this procedure is difficult to perform for a variety of circumstances in the neurological horse.

Table 2. Summary of CSF Findings in Infectious Neurological Diseases
Equine Protozoal Myelitis
Etiological Agent. Many horses likely encounter myelitis-causing protozoal organisms, Sarcocystis neurona or Neospora hughesi, at some point in their life without developing detectable neurologic disease (asymptomatic infection). However little is known about why one horse gets EPM and the other does not. Recent studies indicate that once challenged orally, the parasite is rapidly disseminated in horses and can be isolated from the mesenteric lymph nodes, lung, and liver between 1 and 7 days after infection. Recent studies also indicate that this organism is highly pleomorphic and has antigenic variation which likely accounts for our difficulties in development of diagnostic tests.
Clinical Signs. Clinical signs in affected horses vary from mild muscle wasting or vague lameness to recumbency, convulsions, and death. The variability of clinical signs due to S. neurona makes clinical identification without ancillary testing at times difficult. The protozoa infect the CNS of the horse in low numbers, diffusely, and in several areas, causing insidious and multifocal or diffuse disease. These organisms appear in gray and/or white matter, brain and/or spinal cord. Although onset may be slow and insidious, many horses develop acute neurological dysfunction. Cranial nerve involvement reflects its predilection for the hindbrain in horses. Spinal ataxia can be present and can be symmetrical or asymmetrical. Weakness and muscle atrophy in one or more limbs is common. Muscle atrophy is also associated with cranial nerve dysfunction and most often occurs with temporal-masseter muscle atrophy associated with involvement of the 5th cranial nerve.
Diagnosis. In the original NAHMS study, neurological disease caused by equine protozoal myeloencephalomyelitis (EPM) diagnosis was based on clinical signs alone and no ancillary testing whatsoever was done in 60% of these horses41. Diagnosis and treatment of this disease has only been moderately improved since isolation of the organism Sarcocystis neurona in 1991. Most horses test positive in their serum for EPM, but relatively few actually develop signs of EPM. Irrespective of assay, a positive test from the serum of a horse with clinical signs of EPM does not diagnose the disease. Likewise, cerebrospinal fluid (CSF) can test positive after exposure to disease and these horses may or may not develop signs of EPM. Many false positive tests occur; in fact horses that are vaccinated by EPM vaccine become CSF positive. Acupuncture points have also been proven to be unreliable for diagnosis of EPM. Recent studies have indicated a wider geographic range for Neospora hughesi (or caninum) as a cause of EPM, thus testing for this organism is also necessary to completely rule out EPM as a cause of clinical signs. Other reports indicated that S. neurona can change its major surface proteins thus making testing based on certain of these proteins could be unreliable. The latest information indicates that Elisa's based on the SAG2 protein have a 95.5% and a 92.9% sensitivity and specificity, respectively. Potentially, various other isotype formats such as IgM may indicate acute infection vs. previous exposure.
Treatment. The triazine family of medications, ponazuril (Marquis); dial doser at 5 mg/kg for 28 days. Two months of treatment is recommended. Recent reports indicate that intermittent therapy with ponazuril every 7 days will decrease production of antibodies in the CNS and anectdotal reports indicate that 10 mg/kg is needed. This indirectly indicates the use of this drug as either a preventative therapy for high risk horses (e.g. young horses in training) or 2) possible prevention of recurrence in previously, diagnosed, at risk horses. Folic acid inhibitors are a second option consisting of a combination of trimethoprim-sulfamethoxazole (30 mg/kg) and pyrimethamine at 2.0 mg/kg, SID for 3 days then TMS at 30 mg/kg and pyrimethamine at 1.0 mg/kg for 90 to 180 days depending on response to therapy. Perform CBC every 2-4 weeks to monitor for bone marrow aplasia. Other anti-protozoals consist of nitazoxanide (25 mg/kg for the first week, then 50 mg/kg for the next 3 weeks). This lower dose is recommended because of development of severe diarrhea in some horses that are initially placed on the higher dose. Recent publications have examined intermittent treatment for EPM which include NTZ at 25 mg/kg for 2 days per week or ponazuril at 20 mg/kg once per week. Very low prolonged intermittent dosing at 2.5 mg/kg or 5.0 mg/kg reduced the incidence in a population study.
Viral Encephalitides
Rabies
The World Health Organization marked September 8, 2007 World Rabies Day. Over 50,000 people die each year from this disease. Although most of the death loss occurs in India, Southeast Asia, Africa, and Latin America, eradication will not be possible without protection of pet species in frequent contact with man. Most of the human U.S. cases are from bat bites which, unlike raccoon rabies, is endemic throughout the continent. Even though rabies is rare in horses (compared to other forms of infectious neurological disease), horses are susceptible to fulminate rabies and serve as a public health risk if diseased. Thus identification of potential rabies suspects is essential. The epidemiology and risk factors for disease has always been associated with pastured animals. Given the loss of pasture and the increasing close living proximity of horses to humans, this is likely not a primary risk factor. Indeed, one horse diagnosed in 2006 at a horse show indicated that the event at which the horse became ill had 10,000 spectators. Thus all horses are considered at risk for rabies and thus immunoprophylaxis is recommended.
Clinical Signs. Rabies is primarily transmitted by oral inoculation in the horse. After initial viral replication at the site of infection, transaxonal movement from peripheral nerves to the CNS occurs. Saliva and other body tissues become virus positive at the time of clinical signs. Rabies is one of the most variable neurologic diseases in the horse. Insidious onset is the hallmark of initial clinical signs and reports consist of lameness, colic, dysuria, priapism in addition to neurological disease. Primary clinical signs include anorexia, fever, depression, blindness, mania, dysphasia, hyperesthesia, muscle-twitching, lameness, paresis and ataxia, incontinence, and sudden death. Neurological manifestations have been known to emphasize either brain or spinal cord disease. With the paralytic form, horses experience ascending paralysis and hyperesthesia is common and is manifest by self-mutilation. Behavioral changes occur as in other species with horses presenting as dumb or furious. In the latter horse are maniacal and extremely dangerous.
Diagnosis. Confirmation of rabies virus infection is performed at the respective state Departments of Health (DOH) facilities on FRESH brain. Please send the brain on ice packs to keep cool. Fresh brain testing allows rapid testing by fluorescent antibody testing and the results are read within hours. This test is then usually confirmed by mouse inoculation. It is appropriate to fix one-half of the brain for eventual histological examination and the other half can be sent to one's respective county DOH.
Prevention: Since there is no means of rabies therapy, prevention is the key. Rabies prophylaxis is now considered a core vaccine and it should be administered to horses on an annual basis after the initial series. In foals vaccination should commence at 4-6 months of age. Housing of wild animals as pets is discouraged and surveillance of abnormally acting wildlife is important. Immunoprophylaxis of all domestic carnivores is a must.
Eastern Equine Encephalitis
Eastern equine encephalomyelitis virus is quite active in the U.S. and over 500 EEE cases have been reported in Southeastern horses since 2001. Most of these cases occurred in young horses less than 3 years. Many of these horses were not vaccinated. Recent arrivals to the Southeast represent another high risk group.
Clinical Disease: Disease due to EEE in horses can vary from mild febrile infection to the more common severe, fatal encephalomyelitis. At about five days post exposure, horses experience a high fever with depression and inappetence. Many times these horses have elevated heart rates, even with the depression. There may be an insidious onset of lameness or neurologic signs, but horses generally progress with rapidly deteriorating brain signs. Death can occur within 2-3 days. Horses with EEE frequently show signs of coma, blindness, dementia, head pressing and seizure. At least 75-95% of EEE horses die. If horses survive, many times they are not ever normal mentally and have residual spinal cord abnormalities. A less severe form of the neurological disease rarely does occur. These horses have usually had some vaccination and generally develop mild to moderate forebrain signs with spinal ataxia. An even less common syndrome of generalized febrile disease may also occur and is associated with anorexia, severe depression and high fever. With subclinical exposure, horses may seroconvert with mild lymphopenia. This primarily has been documented with experimental infection.
Diagnosis. During the West Nile virus outbreak, National Veterinary Services Laboratory in Ames developed the IgM capture ELISA for diagnostic testing. This protocol was actually based on testing for EEE and developed by Sahu, et. al. in the 1990's. Since 2001, the most efficient means of EEE confirmatory testing has once again proved to be this format with recent studies performed by NVSL have indicated a high degree of specificity and sensitivity.
Vaccination. Given the high case fatality rate of EEE, there is obviously no proven therapy for EEE and other alphaviruses. Little is known regarding intervention with specific antibody therapy or interferon as has been anecdotally reported effective in WNV treatment. This disease can be solely be prevented by vaccination. Given that killed vaccines are the only products available, short but effective immunity is expected. The primary caveat is vaccination of young horses. Weanlings (starting at 5-6 months of age) must be vaccinated with vaccine injections and then boostering again before mosquito season is a must in endemic regions. Boosters at 4-6 month intervals are highly recommended in the Southeastern region, especially in years when disease activity is reportedly high. Every 4 months is highly recommended in ALL horses that are younger than 3 years of age. In temperate regions, if EEE is identified early in the season by mosquito testing or reporting of clinical disease in mammals, 6 month boostering after primary immunization is necessary to prevent outbreaks in horses. Otherwise, most Northern climates require yearly boosters. ALL horses and foals traveling to the Southeast should be boostered before arrival. If there is sporadic vaccination, then these horses should undergo a primary immunization series two months before shipping so that the second injection is given at least 3 weeks before arrival in the Southeast.
West Nile Virus
West Nile virus (WNV) infection was first diagnosed in horses in the United States in 1999 and is now an important consideration in the differential diagnosis of horses presenting with signs of neurologic disease in all areas of North America since this arbovirus is now endemic in North America. Over 23,000 cases of WNV encephalitis has been reported in U.S. horses with 1,069 cases reported in 2006 (Center for Disease Control and United States Department of Agriculture Websites). This virus is transmitted by mosquitoes, and infrequently by other bloodsucking insects, to horses, human beings, and a number of other mammals from avian hosts, which serve as natural reservoirs for these viruses in nature. Horses and humans are considered to be dead-end hosts of the WNV and the virus is not directly contagious from horse to horse or horse to human. Similarly, indirect transmission via mosquitoes from infected horses is highly unlikely because horses do not circulate a significant amount of virus in their blood.
Early epidemiological studies indicated that vaccination was likely the most effective means of preventing disease in the horse. Vaccination with one of the commercially available licensed vaccines is recommended as core prophylaxis for all horses residing in North America. Given that WNV is now present in South America, vaccination should also be given to horses where the disease occurs. Four licensed vaccines are now available.
While none of the licensed vaccines is labeled for administration to pregnant mares at this time, practitioners have vaccinated pregnant mares due to the risk associated with pregnant mares getting the disease from infected mosquitoes. It has been accepted practice by many veterinarians to administer vaccines to pregnant mares on the assumption that the risk of adverse consequences of WNV infection outweighs any reported adverse effects of use of vaccines in pregnant mares. Booster vaccination of pregnant mares 4 to 6 weeks before foaling may provide an augmented passive colostral protection to their foals. Primary vaccination of foals from vaccinated mares should be started at 5-6 months of age.
Horses that have been naturally infected with the disease that exhibit clinical signs of WNV are likely protected for the remainder of their lives. Consider revaccination only if the immune status of the animal changes the risk for susceptibility to infection. Examples of these conditions would include the long term use of corticosteroids and pituitary adenoma.
Herpesvirus Myeloencephalitis
Herpesvirus myeloencephalitis is caused by an alphavirus that cause neurologic signs due to development of vasculitis, thrombosis, and necrosis of neurological tissue. The virus infects the vascular endothelium of the blood-brain barrier and causes widespread vascular injury. This vascular injury then causes secondary neuronal damage. Herpesvirus myeloencephalitis occurs as clusters and may be associated with a previous respiratory outbreak. The Herpesvirus most associated with neurological manifestation is a type 1 virus (EHV-1) that has a short incubation, reproductive cycle and has the ability to form latent infection. It is presently hypothesized that the specific strain of EHV-1 most associated with neurogical Herpesvirus has only one nucleotide change.
Clinical Signs. Like most infectious neurological diseases, the clinical signs and course of therapy is variable. Primarily this virus causes a symmetrical ataxia and weakness of the pelvic limbs with urinary incontinence, loss of sensation and motor deficits around the tail and perineal area. Gait deficits can be initially minor, resemble lameness and then rapidly progress to recumbence.
Treatment. As usual, most of the therapy for EHV-1 horses is supportive. Horses are bright and alert but have significant impairment of movement. They struggle and injure themselves trying to maintain balance or rise. Support with slinging may be necessary during the worst time of locomotion disorder. Anti-inflammatory therapy with flunixin meglumine and low dose corticosteroids is highly recommended. DMSO is advocated. Supportive therapy with Vitamin E is also pursued. Acyclovir is marketed for treatment of Herpesvirus infections in people and there is interest in the veterinary community in using this drug. There is variable absorption of this product. Work with valcyclovir appears promising. In a pharmacokinetic study, a dose of 40 mg/kg administered three times daily should be sufficient to reach plasma concentration levels that remain 50% above effective concentrations.
Verminous Encephalitis
Verminous encephalitis is rare but does occur in the Midwest and Southeast. Causes to consider specifically include Strongylus vulgaris, Setaria filariids, Halicephalobus gingivalis, and Drachia megastoma, and Hypoderma. Setaria and Strongylus cause brain or spinal cord disease. Signs are ipsilateral and sudden, due to an infarctive process. Halicephalobus and Hypoderma usually are intracranial. Hemorrhage and high numbers of eosinophil and neutrophils occur with verminous CNS disease. There is marked discoloration of the CSF. Verminous infections are usually composed of elevated eosinophil counts with high gamma globulins peripherally. Ivermectin does not cross the blood brain barrier unless there is disruption. Fenbendazole at high doses supposedly can reach the CNS and treatment includes 50 mg/kg for five consecutive days via nasogastric tube. Treat aggressively with fenbendazole, but also include anti-inflammatories and avermeictins should perirenal infection be present with Halicephalobus gingivalis.

Table 3. Update on Reporting of Infectious Neurological Disease