New Veterinary Oncology Therapeutics
Cancer research is advancing at an unprecedented pace, bringing a host of new treatment possibilities.

The diverse expanse of specialized veterinary oncology therapeutics both on the market and on the horizon makes it challenging to remain up-to-date on the latest advances and technologies, yet this information is vital to practices interested in providing the newest cancer treatment options. Immunotherapy is leading the way in human oncology treatment development, and this trend is following in veterinary oncology.
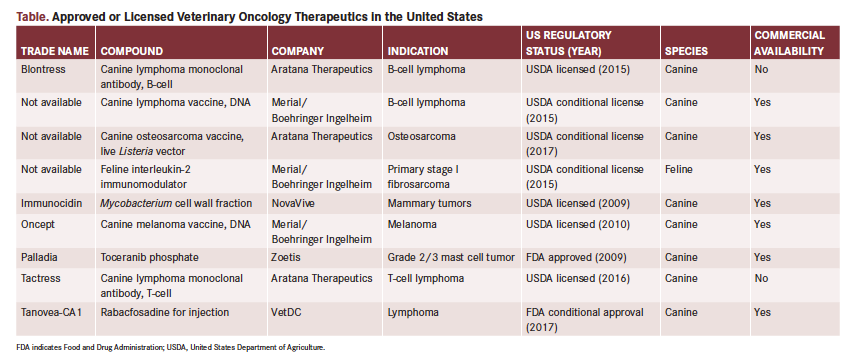
This article briefly summarizes the science, supporting data, regulatory status, and clinical use of several new canine and feline oncology therapeutics, including supportive care therapies that may have a critical impact on our ability to improve quality of life for dogs and cats diagnosed with cancer. Although some of these drugs are in the earlier stages of development, they hold strong potential and are becoming clinically available for patient care. Veterinary oncology thera­peutics of interest in the United States, regulated by either the FDA or the US Department of Agriculture (USDA), are summarized in the Table.
CLINICALLY AVAILABLE ONCOLOGY IMMUNOTHERAPIES
Canine Osteosarcoma Vaccine, Live Listeria Vector (AT-014; Aratana Therapeutics)
What it is: A lyophilized formulation of a modified-live, attenuated, recombinant HER2/neu—expressing strain of Listeria that activates cytotoxic T cells.1,2
Approval: Conditionally licensed by the USDA in December 2017 for the treatment of dogs 1 year or older diagnosed with appendicular osteosarcoma1; administered in a series of 3 doses given 3 weeks apart, with boosters every 6 months.
Clinical data: The nonlyophilized form of this thera­peutic (see next section) was administered to dogs with appendicular osteosarcoma following amputation or limb salvage surgery and chemotherapy (4 doses of carboplatin). In 18 dogs with no evidence of metastatic disease at enrollment that were treated with AT-014, the median disease-free interval was 615 days and median survival time was 956 days. Adverse events were mild to moderate and primarily consisted of fever, lethargy, and nausea/vomiting.3
A field safety study with AT-014 (submitted to the USDA for conditional licensure) revealed the most common adverse events to be lethargy, diarrhea, and fever. Four serious adverse events were observed.2
Aratana is now conducting an extended clinical field study as required by the USDA to progress to full licensure. The vaccine is currently available for purchase at approximately 2 dozen US veterinary oncology practice groups throughout the country that are participating in the extended field study.2,4
Canine Osteosarcoma Vaccine (ADXS31-164; Advaxis Immunotherapies/Aratana Therapeutics)
What it is: A nonlyophilized, frozen form of the USDA conditionally licensed therapeutic AT-014 (discussed above).
Approval: This product is available via clinical trial only and has not been licensed by USDA.
Clinical data: Although the role of HER2/neu expres­sion and targeting has been debated, clinical investiga­tor Nicola Mason, PhD, BVetMed, estimates that 70% to 80% of canine osteosarcoma samples evaluated in her laboratory at the University of Pennsylvania School of Veterinary Medicine stain positively via immunohisto­chemistry using a polyclonal anti-HER2/neu antibody.1
ADXS31-164 is also being employed in a separate multicenter clinical trial evaluating safety and efficacy in dogs with osteosarcoma (target enrollment is 100). This study is funded by Morris Animal Foundation and coordinated by the Comparative Oncology Trials Consortium (part of the National Institutes of Health), with 11 participating sites.1,5
Canine Lymphoma Vaccine, DNA (Merial/Boehringer Ingelheim)
What it is: Xenogeneic murine CD20 DNA therapeutic vaccine for use in dogs with B-cell lymphoma.
Approval: Conditionally licensed by the USDA in 2015 for the therapeutic immunization of dogs diagnosed with large B-cell lymphoma upon achieving remission through chemotherapy.
Clinical data (personal communication, Merial/Boeh-ringer Ingelheim, May 2018): No peer-reviewed data have been published to date. A preclinical safety study was performed in 45 dogs, the results of which have not yet been published.
A multicenter international randomized controlled safety and efficacy study in dogs with large B-cell lymphoma was initiated in April 2014. In this study, remission was induced with a CHOP (cyclophospha­mide, doxorubicin, vincristine, prednisone)—based chemotherapy protocol and followed by vaccination (4 doses given at 2-week intervals). More than 60 dogs have been enrolled; results are pending.
Another multicenter randomized controlled study in dogs with large B-cell lymphoma concurrent with CHOP-based chemotherapy, which was started in April 2016, is evaluating humoral CD20 antibody response. Enrollment of 24 dogs is targeted; results are pending.
Feline Interleukin-2 (IL-2) Immunomodulator Vaccine (Merial/Boehringer Ingelheim)
What it is: Recombinant canarypox virus (ALVAC) expressing feline IL-2.
Approval: Approved by the European Medicines Agency in 2013 (Oncept IL-2); conditionally licensed by the USDA in 2015 to delay postsurgical recurrence of feline fibrosarcoma in adult cats with stage I disease.6
Clinical data: Adjuvant treatment of feline injection-site sarcomas with this immunotherapy in conjunction with surgery and brachytherapy has been described.7 Treatment was well tolerated, with adverse events limited to mild local reactions. The treatment (low dose) group had a significantly longer median time to relapse than did the reference group.7
A multicenter randomized controlled trial to further evaluate the efficacy and safety of this vaccine as an adjunct to surgical resection of primary (first occur­rence) stage I feline fibrosarcoma was initiated in April 2015. The enrollment target is 75 cats; results are pending (personal communication, Merial/Boehringer Ingelheim, May 2018).
POTENTIAL ONCOLOGY IMMUNOTHERAPEUTIC TARGETING
PD-1/PD-L1
What it is: Inhibition of T-cell checkpoint molecules such as PD-1 and PD-L1, which has produced signifi­cant responses in advanced human tumors (melanoma, renal cell carcinoma, and non—small cell lung cancer).8
Clinical data: Recent studies have reported expression of PD-L1 in several canine malignant tumors, includ­ing oral melanoma, osteosarcoma, hemangiosarcoma, mast cell tumor, mammary and prostate adenocarci­noma, and lymphoma.9,10
On the horizon: New immunotherapies in veterinary oncology will likely target this pathway given the success noted by such therapies for various human cancers. Kindred Biosciences11 and Zoetis (with the recent acquisition of Nexvet) have indicated that they are working on checkpoint-inhibiting monoclonal antibodies for dogs.
CD20
What it is: A well-recognized therapeutic target in human B-cell lymphoma, offering hope that immuno­therapy directed against CD20 will prove beneficial in the treatment of dogs with B-cell lymphoma.
Clinical data: Although an anti-CD20 monoclonal antibody is fully licensed (Blontress, Aratana), the company has stated that Blontress is not as specific to the CD20 target as expected.12 No peer-reviewed data are available on this therapeutic to date, nor is it avail­able commercially.
On the horizon: Elanco13 and Kindred Biosciences11 have indicated they are working on canine anti-CD20 monoclonal antibodies for lymphoma.
CD52
What it is: Documented therapeutic target in certain human T-cell lymphoid malignancies; target not yet identified on canine lymphoid cells.
Clinical data: Although an anti-CD52 monoclonal antibody is fully licensed (Tactress, Aratana), the drug manufacturer has stated that Tactress is not as specific to the CD52 target as expected.12 No peer-reviewed data are available on this therapeutic to date, and it is not commercially available.
On the horizon: Whether CD52 becomes an active target for future veterinary therapeutics remains to be determined.
CYTOTOXIC CHEMOTHERAPEUTICS AND INTRATUMORAL THERAPIES
Rabacfosadine (Tanovea-CA1; VetDC)
What it is: Prodrug of the nucleotide analogue 9- (2-phosphonylmethoxyethyl) guanine (PMEG); effec­tively loads lymphoid cells while reducing levels of PMEG in plasma and target organs of toxicity.
Approval: Conditionally approved by the FDA in January 2017 for the treatment of lymphoma in dogs; became available to veterinarians in spring 2017.14
Clinical data: Tanovea-CA1 has been or is being eval­uated in clinical studies involving over 500 dogs (personal communication, VetDC, May 2018). For example, in a phase 1/2 trial in 38 dogs with non-Hodgkin lymphoma using different dose schedules, 30 (79%) dogs receiving Tanovea-CA1 monotherapy achieved clinical remission (23 [61%] complete remis­sion [CR] and 7 [18%] partial remission [PR]). Median first remission duration for all dogs was 128 days.15 In other studies, the drug:
- Was used with prednisone in dogs with naïve and relapsed lymphoma (n = 74).16
- Was administered at different doses in dogs with naïve and relapsed lymphoma (n = 63).17
- Was alternated with doxorubicin in dogs with naïve lymphoma (n = 51).18
- Was given at different doses in dogs with relapsed lymphoma that underwent 1 prior doxorubicin-con­taining regimen (n = 50).19
- Was evaluated for efficacy in dogs with cutaneous T-cell lymphoma (n = 11)20 and multiple myeloma (n = 14).21
- Is being evaluated for use in cats with lymphoma.22
Assessing all studies that have been evaluated for reasonable expectation of efficacy as part of FDA condi­tional approval, the overall response rate in dogs with lymphoma is 77% (45% CR, 32% PR). Tanovea-CA1 has demonstrated reasonable expectation of efficacy in dogs with lymphoma that are naïve to chemotherapy, as well as in those that have relapsed or are refractory to conventional chemotherapy (personal communica­tion, VetDC, May 2018).23
VetDC has communicated that the pivotal efficacy study for Tanovea-CA1 was to be initiated in fall 2018, with expected enrollment of 135 dogs with naïve and relapsed lymphoma (100 treated, 35 placebo) (personal communication, VetDC, May 2018).
On the horizon: The best clinical use and fit for Tanovea-CA1 will be defined as more clinical expe­rience and published data become available. Based on currently available information, Tanovea-CA1 is a potential option for clients who desire a single agent treatment with fewer visits than conventional multi-agent CHOP requires. Additionally, it has potential use as a second or third line of therapy following CHOP and/or MOPP (mechlorethamine, vincristine, procar­bazine, prednisone) multiagent protocols.
Tigilanol Tiglate (EBC-46; QBiotics)
What it is: Novel anticancer pharmaceutical protein kinase C activator, isolated from the seeds of Fontainea picrosperma (blushwood tree)24,25 and used for intratu­moral injection; proposed FDA Center for Veterinary Medicine and European Medicines Agency indication: nonmetastatic skin mast cell tumors in dogs (personal communication, QBiotics, May 2018).
Approval: FDA application was expected in August 2018; European Medicines Agency centralized submission was planned for September 2018.
Clinical data: In Australian clinical trials in which more than 100 dogs with mast cell tumors were treated with EBC-46, 79.7% had CR following a single injection, and 72.9% were tumor free in 12 to 24 months (data collection ongoing; personal communication, QBiotics, May 2018).
Efficacy programs have been completed, including a pivotal clinical efficacy trial in the United States (11 sites, n = 122 dogs), the results of which have not yet been published.
ONCOLOGY SUPPORTIVE THERAPEUTICS
Capromorelin Oral Solution
(Entyce; Aratana Therapeutics)
What it is: An orally active small molecule that mimics the action of ghrelin, which causes growth hormone secretion and appetite stimulation; has significant potential to treat inappetence related to chemo­therapy and/or underlying cancer in dogs and possibly cats.
Approval: Approved by the FDA in May 2016 for appetite stimulation in dogs; became avail­able to veterinarians in fall 2017.
Clinical data: Capromorelin oral solution dosed at 3 mg/kg/day has been shown to increase food intake and weight gain in both healthy labora­tory and in appetent client-owned dogs.26-28
The drug has demonstrated a wide margin of safety, being well tolerated at daily doses up to 40 mg/kg for 12 consecutive months.29
When administered to healthy dogs for 7 days, capromorelin produced increased insulin-like growth factor 1 (IGF-1) concentrations on day 1 that were sustained through day 7.26 IGF-1 is an important anabolic growth factor for regulating muscle hypertro­phy.30 Via this mechanism, capromorelin may be able to help reverse muscle atrophy/loss that occurs due to chronic medical conditions.
Anamorelin, a therapeutic with a similar mechanism, has been studied in the treatment of cancer-related cachexia in human non-small cell lung cancer. Results showed that anamorelin significantly increased lean body mass and may be a valid treatment option for human patients with cancer anorexia and cachexia.31
Capromorelin has been demonstrated to cause increases in IGF-1 and increased food intake and body weight in cats.32
On the horizon: Aratana is also developing a feline-spe­cific formulation with additional studies pending.
Mirtazapine Transdermal Ointment
(Mirataz; Kindred Biosciences, Inc)
What it is: A generic tetracyclic antidepressant for humans with ancillary properties that include anxi­olytic, sedative, antiemetic, and appetite stimulant effects.
Approval: Approved by the FDA in May 2018 for managing weight loss in cats.33 The transdermal oint­ment is administered topically at a dose of 2 mg/cat/ day for 14 days via a 1.5-inch ribbon on the inner pinna.34 It is available commercially.
Clinical data: The pivotal efficacy study included 177 cats, 83 treated with Mirataz and 94 with vehicle control. The primary efficacy endpoint was percent­age change in body weight at 14 days. At study end, the mean percentage increase in body weight from day 1 was 3.94% in the mirtazapine group compared with 0.41% in the vehicle control group.
The difference between the 2 groups was significant (P < .0001).34
The most common adverse events reported in cats (>10%) treated with Mirataz included vocalization (11.3%), vomiting (11.3%), and erythema at the appli­cation site (10.4%).34Canine-Specific Crofelemer (Canalevia, Jaguar Animal Health and Elanco)
What it is: Active pharmaceutical ingredient isolated and purified from the Croton lechleri tree that contains antisecretory properties35; not absorbed systemically at the therapeutic dose but acts locally in the gastroin­testinal tract.35
Approval: In February 2017, Jaguar announced an agreement to license, develop, and commercialize Canalevia jointly with Elanco. Jaguar has retained commercial responsibility for the chemotherapy-in­duced diarrhea indication of Canalevia in dogs, which has received minor use and minor species designation from the FDA and which the company expects will be the first indication available commercially (timeline not specified).36
Clinical data: None available.
On the horizon: Canalevia is an oral, enteric-coated, twice-daily formulation being developed for the treat­ment of acute and chemotherapy-induced diarrhea in dogs.35,36
Disclosures
Within the past 3 years, Dr. Johannes has the following disclosures relevant to this article: Aratana Therapeutics (advisory board, paid consultant, clinical trial); Jaguar Animal Health (clinical trial); Merial/Boehringer Ingelheim (research grant); NovaVive (paid consultant, research grant); QBiotics (advisory board, paid consultant); VetDC (paid consultant); Zoetis (advisory board, research grant).
REFERENCES
1. Burns K. Vaccine holds promise for treating osteosarcoma in dogs. American Veterinary Medical Association website. avma.org/News/JAVMANews/Pages/171201b.aspx. Published November 15, 2017. Accessed November 27, 2018.2.
2. Aratana Therapeutics granted conditional license for a canine osteosarcoma therapeutic [press release]. Leawood, KS: Aratana Therapeutics, Inc; December 20, 2017. prnewswire.com/news-releases/aratana-therapeutics-granted-conditional-license-for-a-canine-osteosarcoma-therapeutic-300573668.html. Accessed November 27, 2018.
3. Mason NJ, Gnanandarajah JS, Engiles JB, et al. Immunotherapy with a HER2-targeting Listeria induces HER2-specific immunity and demonstrates potential therapeutic effects in a phase I trial in canine osteosarcoma. Clin Cancer Res. 2016;22(17):4380-4390. doi: 10.1158/1078-0432.CCR-16-0088.
4. AAHSD004607: additional field safety testing of canine osteosarcoma vaccine, live Listeria vector. AVMA website. ebusiness.avma.org/aahsd/study_search_detail.aspx?sid=4607. Accessed November 27, 2018.
5. Current open clinical trials. National Cancer Institute Center for Cancer Research website. ccr.cancer.gov/Comparative-Oncology-Program/pet-owners/trials. Accessed November 27, 2018.
6. USDA grants conditional license for first immunotherapy to delay the recurrence of fibrosarcomas in cats. Todays Vet Pract. 2015;5(3):15.
7. Jas D, Soyer C, De Fornel-Thibaud P, et al. Adjuvant immunotherapy of feline injection-site sarcomas with the recombinant canarypox virus expressing feline interleukine-2 evaluated in a controlled monocentric clinical trial when used in association with surgery and brachytherapy. Trials Vaccinol. 2015;4:1-8.
8. Regan D, Guth A, Coy J, Dow S. Cancer immunotherapy in veterinary medicine: current options and new developments. Vet J. 2016;207:20-28. doi: 10.1016/j.tvjl.2015.10.008.
9. Maekawa N, Konnai S, Okagawa T, et al. Immunohistochemical analysis of PD-L1 expression in canine malignant cancers and PD-1 expression on lymphocytes in canine oral melanoma. PLoS One. 2016;11(6):e0157176. doi: 10.1371/journal.pone.0157176.
10. Shosu K, Sakurai M, Inoue K, et al. Programmed cell death ligand 1 expression in canine cancer. In Vivo. 2016;30(3):195-204.
11. Pipeline snapshot. Kindred Bio website. kindredbio.com/pipeline/. Updated November 7, 2018. Accessed November 27, 2018.
12. Aratana Therapeutics provides product updates [press release]. Kansas City, KS: Aratana Therapeutics, Inc; September 24, 2015. aratana.investorroom.com/2015-09-24-Aratana-Therapeutics-Provides-Product-Updates. Accessed November 27, 2018.
13. Rue SM, Eckelman BP, Efe JA, et al. Identification of a candidate therapeutic antibody for treatment of canine B-cell lymphoma. Vet Immunol Immunopathol. 2015;164(3-4):148-159. doi: 10.1016/j.vetimm.2015.02.004.
14. VetDC receives FDA conditional approval of TANOVEA®-CA1, the first new animal drug for treating lymphoma in dogs [press release]. Fort Collins, CO: VetDC, Inc; January 3, 2017. vet-dc.com/news/vetdc-receives-fda-conditional-approval-tanovea-ca1-first-new-animal-drug-treating-lymphoma-dogs/. Accessed November 27, 2018.
15. Vail DM, Thamm DH, Reiser H, et al. Assessment of GS-9219 in a pet dog model of non-Hodgkin's lymphoma. Clin Cancer Res. 2009;15(10):3503-3510. doi: 10.1158/1078-0432.CCR-08-3113.
16. Thamm D, Morges MA, Clifford CA, et al. Rabacfosadine and prednisone: efficacy of a Q21 day administration scheduled in canine lymphoma. Abstract presented at the 2015 American College of Veterinary Internal Medicine Forum; Indianapolis, IN; June 13-15, 2015.
17. Thamm D, Clifford C, Vickery K, et al. Rabacfosadine for canine lymphoma: efficacy and adverse event profiles of two different doses. Abstract presented at the 2015 Veterinary Cancer Society Conference; Tysons, VA;73.
18. Thamm DH, Vail DM, Post GS, et al. Alternating rabacfosadine/doxorubicin: efficacy and tolerability in naïve canine multicentric lymphoma. J Vet Intern Med. 2017;31(3):872-878. doi: 10.1111/jvim.14700.
19. Saba CF, Vickery KR, Clifford CA, et al. Rabacfosadine for relapsed canine B-cell lymphoma: efficacy and adverse event profiles of 2 different doses. Vet Comp Oncol. 2018;16(1):E76-E82. doi: 1111.vco.12337.
20. Morges MA, Burton JH, Saba CF, Vail DM, Burgess KE, Thamm DH. Phase II evaluation of VDC‐1101 in canine cutaneous T‐cell lymphoma. J Vet Intern Med. 2014;28(5):1569-1574. doi: 10.1111/jvm.12429.
21. Thamm DH, Vail DM, Kurzman ID, et al. GS-9219/VDC-1101 — a prodrug of the acyclic nucleotide PMEG has antitumor activity in spontaneous canine multiple myeloma. BMC Vet Res. 2014;10:30. doi: 10.1186/1746-6148-10-30.
22. VetDC product pipeline. VetDC website. vet-dc.com/products/. Accessed November 30, 2018.
23. Tanovea®-CA1 (rabacfosadine for injection) [package insert], Fort Collins, CO: VetDC. December 2016.
24. Boyle, GM, D’Souza MM, Pierce CJ, et al. Intra-lesional injection of the novel PKC activator EBC-46 rapidly ablates tumors in mouse models. PLoS ONE. 2014;9(10):e108887. doi: 10.1371/journal.pone.0108887.
25. Barnett, ME, Broit N, Yap PY, et al. Optimising intratumoral treatment of head and neck squamous cell carcinoma models with the diterpene ester tigilanol tiglate [published online April 18, 2018]. Invest New Drugs. 2018. doi: 10.1007/s10637-018-0604-y.
26. Zollers B, Rhodes L, Smith RG. Capromorelin increases food consumption, body weight, growth hormone, and sustained insulin-like growth factor 1 concentrations when administered to healthy adult beagle dogs. J Vet Pharmacol Ther. 2017;40(2):140-147. doi: 10.1111/jvp.12344.
27. Zollers B, Wofford JA, Heinen E, Huebner M, Rhodes L. A prospective, randomized, masked, placebo-controlled clinical study of capromorelin in dogs with reduced appetite. J Vet Intern Med. 2016;30(6):1851-1857. doi: 10.1111/jvim.14607.
28. Zollers B, Rhodes, L. Heinen E. Capromorelin oral solution (ENTYCE) increases food consumption and body weight when administered for 4 consecutive days to healthy adult beagle dogs in a randomized, masked, placebo-controlled study. BMC Vet Res. 2017;13(1):10. doi: 10.1186/s12917-016-0925-z.
29. Zollers B, Huebner M, Armintrout G, Rausch-Derra LC, Rhodes L. Evaluation of the safety in dogs of long-term, daily oral administration of capromorelin, a novel drug for stimulation of appetite. J Vet Pharmacol Ther. 2017;40(3):248-255. doi: 10.1111/jvp.12358.
30. Timmer LT, Hoogaars WMH, Jaspers RT. The role of IGF-1 signaling in skeletal muscle atrophy. Adv Exp Med Biol. 2018;1088:109-137. doi: 10.1007/978-981-13-1435-3_6.
31. Temel JS, Abernethy AP, Currow DC, Friend J, Duus EM, Yan Y, Fearon KC. Anamorelin in patients with non-small-cell lung cancer and cachexia (ROMANA 1 and ROMANA 2): results from two randomized, double-blind, phase 3 trials. Lancet Oncol. 2016;17(4):519-531. doi: 10.1016/S1470-2045(15)00558-6.
32. Wofford JA, Zollers B, Rhodes L, Bell M, Heinen E. Evaluation of the safety of daily administration of capromorelin in cats. J Vet Pharmacol Ther. 2018;41(2):324-333. doi: 10.1111/jvp.12459.
33. Kindred Biosciences receives FDA approval of Mirataz (mirtazapine transdermal ointment) for the management of weight loss in cats [press release]. San Francisco, CA: Kindred Biosciences, Inc; May 7, 2018. kindredbio.com/frontpage/kindred-biosciences-receives-fda-approval-of-mirataz-mirtazapine-transdermal-ointment-for-the-management-of-weight-loss-in-cats/. Accessed November 27, 2018.
34. Mirataz (mirtazapine transdermal ointment) [package insert]. Burlingame, CA: Kindred Biosciences, Inc; revised May 2018.
35. Jaguar completes study in dogs with chemotherapy-induced diarrhea with commercial formulation of Canalevia [press release]. San Francisco, CA: Jaguar Animal Health, Inc; July 1, 2015. businesswire.com/news/home/20150701005278/en/Jaguar-Completes-Study-Dogs-Chemotherapy-Induced-Diarrhea-Commercial#.Vc4B8ZNViko. Accessed November 27, 2018.
36. Jaguar Animal Health, Elanco enter global collaboration for development, co-promotion of Canalevia [press release]. San Francisco, CA: Animal Health, Inc; January 31, 2107. elanco.com/news/press-releases/jaguar-press-release. Accessed November 27, 2018.
Dr. Johannes is an assistant professor of oncology at the Iowa State University College of Veterinary Medicine. His practice experience includes primary care, specialty care, and academic settings. His areas of research interest include oncology therapeutic development, immunotherapeutics, and effective manage­ment of treatment-related adverse effects.
