Oncology: An ounce of surgical planning worth a pound of long-term treatment
It is well known that the first attempt at surgery is the one most likely to provide control and/or "cure" of the tumor.
It is well known that the first attempt at surgery is the one most likely to provide control and/or "cure" of the tumor.
Surgeries that are not well planned may jeopardize the long-term prognosis of the patient. There are several axioms that have been used to define surgical oncology, including "measure twice, cut once," "go wide, go deep" and "the tip of the iceberg."
Surgical oncology is not always for the faint of heart. The temptation of doing a less-aggressive surgery due to concerns over reconstruction should be avoided. Pre-surgical planning is crucial to the success of surgery. The first consideration should be how to remove the tumor with a wide margin. Once that is determined, then the decision can be made regarding the best method for closure.
Reconstructive techniques such as grafts, flaps and implants are sometimes needed to close a wound. Tension-relieving techniques prior to surgery (skin stretchers) or following surgery (tension relieving sutures or buttons) can be used for locations that may have delayed healing due to tension on the incision. In some cases it may be appropriate to allow a wound to heal by second intention.
When excising a tumor, it is recommended that the tumor and surrounding normal tissue margins be removed en bloc. Incision into the tumor can lead to contamination of the surgical field, thus increasing the risk of recurrence and complicating any further therapy.
One of the most dangerous phrases used in surgical oncology is "it shelled right out." There may be a capsule surrounding the tumor that is actually a pseudocapsule comprised of compressed normal and tumor cells. When a tumor is shelled out, the pseudocapsule and tumor cells are left behind.
Care should be taken when placing drains as the entire drain tract can potentially be contaminated with tumor cells. If a drain has been placed, it should be located such that it can be easily re-excised or included in the radiation field. The same principle also holds true for a biopsy tract.
The rule of thumb has been to take a 3-5 cm margin of normal tissue when excising a malignant tumor. In reality, this is not always feasible. The required margin may depend on the tumor type, location and size. The deep margin often is the most difficult to obtain and, if it is not possible to obtain a wide, deep margin, then at least one intact fascial plane below the tumor should be included.
If it is suspected that radiation therapy will be required post-operatively, it may be helpful to speak with the radiation oncologist prior to surgery because there are surgical considerations that may affect the efficacy of radiation therapy. Orientation of the scar is important as radiation travels in a straight line. If the scar is oriented such that there is a significant curve (i.e., over the thorax or lumbar region), then radiation therapy planning becomes more difficult and treatment may be suboptimal.
Strategically placed hemoclips may facilitate more accurate identification of the tumor extent for radiation-therapy planning.
Although all radiation oncologists do not use pre-operative radiation therapy routinely, some cases may benefit from the option.
Biopsy submission
Further treatment recommendations are made based on the histopathology report. If the report is inaccurate or incomplete, then any further recommendations that are made are subject to error. Proper submission of the specimen cannot be overemphasized. Offering clients the option of not submitting a specimen for biopsy is dangerous. Charges for specimen submission should be part of the surgical package fee.
Saving money by not obtaining a microscopic description is also discouraged because there can be valuable information in the description regarding the potential behavior of a tumor. A full history should be provided to the pathologist to help correlate the diagnosis with the clinical picture. One of the most common complaints from pathologists is that they do not receive any history or have an incomplete history that hinders their ability to provide an accurate report.
As a rule, the entire tumor should be submitted to the pathologist, because tumors are heterogeneous in nature. Small samples may not be representative of the entire specimen. Submission of a portion of a tumor that contains only necrosis or inflammation may lead to the misdiagnosis of a benign lesion.
Different regions of a tumor can have more malignant features that can be important to identify because the expected behavior of a tumor would be based on the most malignant features.
In most cases, pathology labs provide clinics only with small specimen jars to submission samples in. The lab may be able to provide bigger containers for larger specimens, but it is possible to use other sealable containers, such as Tupperware for these samples. Formalin cannot penetrate into large samples but fixation can be improved by making partial slices into the tumor at 1-in. intervals to allow formalin to penetrate into the specimen. Larger samples can be fixed for 24 hours in formalin and then transported to the lab wrapped in formalin-soaked paper towels in a sealed container.
Submission of only a portion of the tumor compromises margin evaluation. If the margins cannot be determined due to an incomplete submission, it may be necessary to recommend further local therapy that can result in higher costs for the owner as well as additional morbidity for the patient.
If possible, the margins should be marked for the pathologist so that it can be determined which margin (i.e., deep or lateral) is incomplete or narrow. Methods of marking the margins can be simple (India ink) or more complex (5-dye system) depending on the situation. Knowing which margin is narrow or incomplete can help determine if a re-excision would provide an adequate margin.
When a pathologist evaluates the margins, four or five representative margins typically are evaluated (i.e. lateral, medial, cranial, caudal and deep). Note that tumors are not spherical so that the actual margins can vary depending on which area is sampled. Margins are often reported as incomplete, narrow, wide or radical. Pathologists can also "quantify" the margins (i.e. 1 mm or 1 cm) to better help make treatment decisions. A tumor excised with a 1-mm margin may be reported as completely excised but likely requires additional treatment due to the narrowness of the margin.
When determining if a margin is adequate, consider the size of the tumor and the tumor type. Reported margins can be slightly underestimated as tissue has the tendency to shrink once it is placed in formalin.
Discussion of grading
Several tumors have accepted grading schemes that allow the pathologist to assign a grade to the tumor (i.e. mast-cell tumors, soft-tissue sarcomas). Further treatment may be recommended for high-grade tumors. Features that tend to be most important in determining the degree of malignancy include mitotic index, degree of differentiation, degree of necrosis and presence or absence of lymphatic and vascular invasion. Even for those tumors that do not have an accepted grading scheme, it may be possible to predict the behavior of a tumor based on evaluation of these features.
At some point you may be faced with a diagnosis that does not fit the clinical picture. It is up to you to question these cases and look for additional information. The first step usually is to speak with the pathologist about the case to see if the discrepancy can be resolved. If there are still unanswered questions, then having the biopsy reviewed by a different pathologist (or several), requesting special stains or immunohistochemistry (IHC) and/or obtaining additional biopsies, may be indicated.
There also are cases where the pathologist is not able to give a specific diagnosis, given the undifferentiated nature of the tumor. In these cases, the pathologist should be contacted to determine if there are additional special stains (i.e. Toluene blue) or IHC that may be helpful in making a definitive diagnosis. The basis of IHC is the assumption that malignant cells have cellular markers that also are present in normal cells of the same histologic type.
The pathologist should be able to suggest which marker(s) would be appropriate to request.
In many cases, a panel of IHC markers is more helpful than a single marker. There are additional charges for IHC and based on the number and type of markers needed.
Turnaround time of IHC can vary, based on the lab and marker, but generally is between five and 10 business days. More than one set of markers may be required if the initial set is negative.
A positive result is useful, but note that poorly differentiated tumors may have lost normal cellular markers so that a negative result does not necessarily rule out a particular tumor type.
Post-operative therapy
If a margin is incomplete or narrow, then additional surgery or radiation therapy can be considered for local control. Re-excision of the scar typically is preferred over radiation therapy. Second surgeries are less expensive, require fewer visits and may be potentially more effective than radiation therapy.
Radiation therapy may be an option when additional surgery is not possible. The exact protocol will depend on the tumor type as well as the radiation oncologist, but treatment protocols typically are between 15 and 20 treatments over three to four weeks.
Success rates will depend on the tumor type, size and location. The cost can vary between facilities, although an average cost for full-course radiation therapy would be between $3,500 and $5,000.
Radiation therapy would not be started until the surgical site has healed, as radiation therapy can slow the healing process. In most cases, radiation therapy should be started two weeks to six weeks post-operatively in order to minimize the risk of tumor repopulation.
For those lesions that heal by second intention, radiation therapy needs to be delayed until the wound is closed.
For those tumors that have a high risk of metastatic or for those patients that already present with metastatic disease, adjuvant chemotherapy may be recommended.
The chemotherapy protocol is tailored specifically to the tumor type and the individual patient.

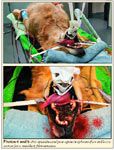
Chemotherapy can slow the healing process as well as increase the risk of infection, so follow-up chemotherapy generally is started at the time of suture removal, provided that the healing process is complete.


However, if the patient is compromised due to the cancer, chemotherapy can be started earlier at the discretion of the clinician.
As with any therapy, it is better to institute treatment early for the best chance of success.
SUGGESTED READING
- Withrow SJ: Biopsy Principles, in Withrow SJ, MacEwen EG (eds): Small Animal Clinical Oncology, Philadelphia, 2001, pp 63-69.
- Ogilvie GK and Moore AS. Biopsy: Theory and Practice in Managing the Veterinary Cancer Patient: A Practice Manual, Trenton, 1995, pp1-5.
- Withrow SJ: Surgical Oncology, in Withrow SJ, MacEwen EG (eds): Small Animal Clinical Oncology, Philadelphia, 2001, pp 70-76.
- Power BE and Dernell SD. Tumor Biology and Pathology. Clinical Techniques in Small Animal Practice 1998; 13:4-9.
- Ehrhart N. Principles of Tumor Biopsy. Clinical Techniques in Small Animal Practice 1998; 13:10-16.
- Dernell SD and Withrow SJ. Preoperative Patient Planning and Margin Evaluation. Clinical Techniques in Small Animal Practice 1998; 13:17-21.
Dr. Cronin earned her DVM degree from Cornell University in 1990. She completed an internship at the Animal Medical Center in New York and a medical oncology residency at North Carolina State University. She is a diplomate of the American College of Veterinary Internal Medicine in the specialty of oncology. After completing her residency, she was lecturer at the University of Pennsylvania Veterinary Teaching Hospital and a medical oncologist at Angell Memorial Animal Hospital in Boston. In 2001, she co-founded the New England Veterinary Oncology Group in Waltham, Mass.
Newsletter
From exam room tips to practice management insights, get trusted veterinary news delivered straight to your inbox—subscribe to dvm360.