Polyuria and polydipsia: streamlining your veterinary diagnostics
Understanding the mechanisms involved with PU/PD, in addition to obtaining a careful medical history, can help clinicians select the appropriate diagnostic tests.
Lightspruch / stock.adobe.com

Many veterinarians see pets with polyuria (PU) and polydipsia (PD) every day. But are our workups as efficient as they could be? Which tests provide the most useful information? At the Fetch dvm360® virtual conference, Heather Kvitko-White, DVM, DACVIM (SAIM), an internist and founder of KW Veterinary Consulting, offered important tips for working through PU/PD.
What factors affect water intake and urination?
The first step in diagnosing PU/PD is defining the condition. What constitutes “normal” water intake is variable, Kvitko-White said, but a range between 40 and 60 ml/kg/day is generally considered acceptable. PD is diagnosed when drinking exceeds 100 ml/kg/day.
Of course, activity level and ambient temperature (heat and humidity) and other factors impact water drinking. For example, food plays a role. Dry kibble tends to make pets drink more water, but so can certain prescription diets. For example, some bladder stone–dissolution diets are higher in sodium, which causes increased drinking. Kvitko-White also noted that high-protein canned food for cats can increase thirst. So, it’s important to ask about the pet’s normal diet when trying to determine whether water intake is excessive. Household environment can also affect drinking behavior, Kvitko-White warned. If 1 cat in a multicat household is being bullied away from the water bowl, that cat may be seen drinking more water when it’s accessible, which can make the owner believe the pet is drinking excessively.
Aside from a list of illnesses, other factors that may cause pets to drink more water include stress (due to increased systemic cortisol levels), nausea, fever, pain, and certain drugs, including corticosteroids.
As with drinking, urine output can be somewhat variable. Urine output of 1 to 2 ml/kg/day is considered normal, and PU is defined as urination that exceeds 2 ml/kg/hr. However, definitively assessing PU is limited because catching all the urine a dog or cat makes over the course of the day is unrealistic.
Kvitko-White also noted, “When we think about PU/PD, it’s important to differentiate whether the issue is drinking too much water or urinating too much.” It makes sense that excessive water intake results in increased urination. But excessive water loss through PU can cause dehydration, which triggers increased drinking. According to Kvitko-White, a key factor to remember is that most of the common differentials for PU/PD begin with PU, which then triggers PD as a response.
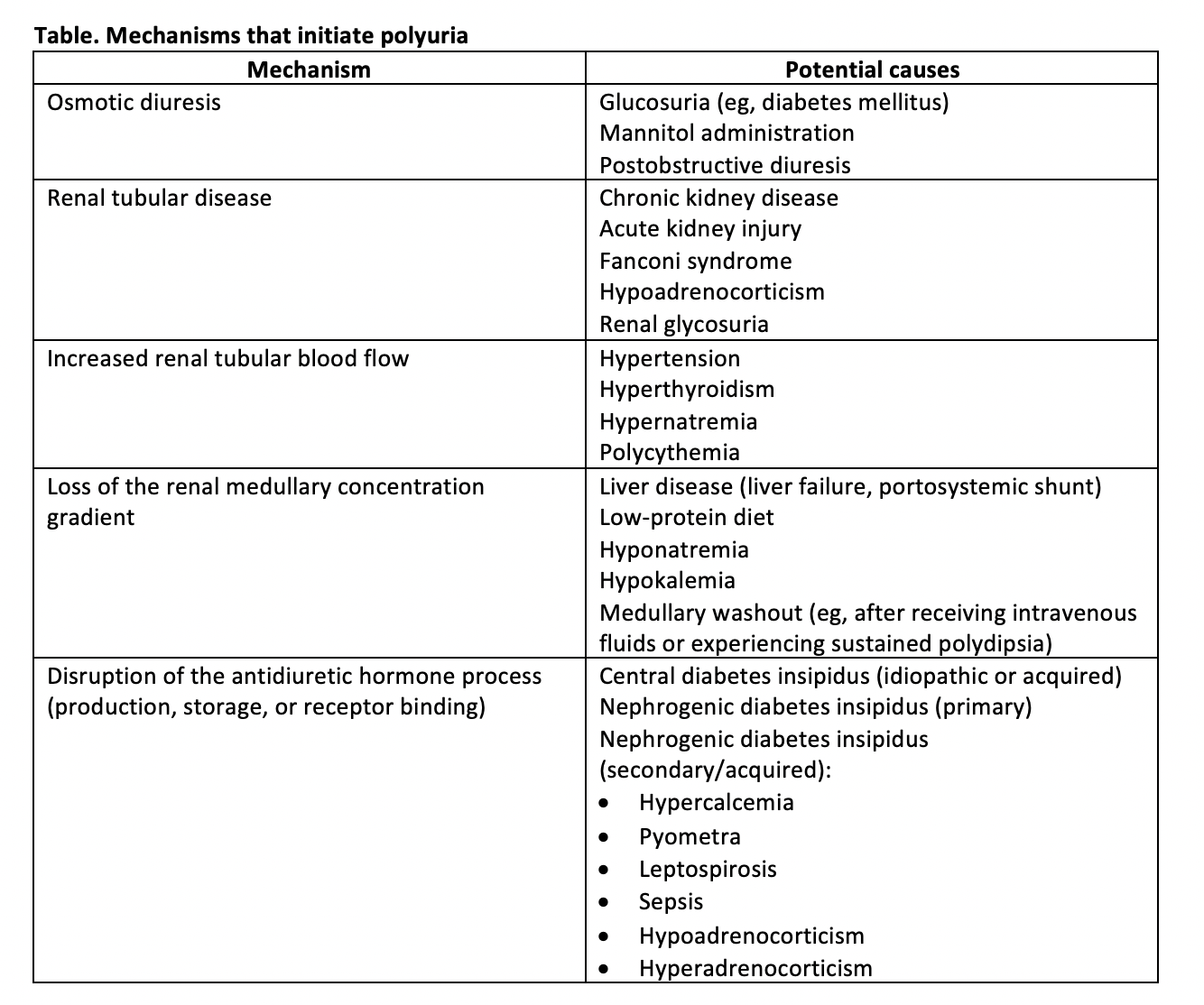
It may simplify one’s clinical approach to consider that there are only 5 main pathophysiologic mechanisms that initiate PU (Table). Understanding the connection between these mechanisms and the diseases that cause them can help clinicians reason through the diagnostic process.
Table 1
Verifying the medical history
When reviewing medical history with a pet owner, it isn’t always easy to verify that what they’re reporting is actually PU/PD versus another situation, such as urinary accidents secondary to lower urinary tract disease. For example, a pet that is urinating more frequently, but not urinating larger volumes,probably doesn’t have PU/PD. Additionally, many of the common clinical signs of PU/PD can overlap with those of other conditions. Leaking urine while sleeping, increased urination at night (including urinating on the pet’s bed or waking the owner to go outside during the night), and having frequent urinary accidents can certainly occur with PU/PD, but they can also occur with lower urinary tract disease and other causes of abnormal urination.
Kvitko-White cautioned that there are some important distinctions that shouldn’t be confused or overlooked. “Lower urinary tract disease does not cause PU/PD unless there’s renal involvement, which is generally rare. Also, in an animal with PU/PD, you shouldn’t see any signs of lower urinary tract discomfort or straining. So, remember that lower urinary tract disease is not a differential for PU/PD,” she advised. Clues like these can help improve the accuracy of the medical history.
Pet owners should also be questioned about any medications the pet is receiving, as common medications such as diuretics and corticosteroids can increase water drinking and urination. Kvitko-White warned that topical, inhaled, and ophthalmic corticosteroid medications can undergo enough systemic absorption to cause increased drinking, so these preparations shouldn’t be dismissed when trying to decide whether a medication might be responsible for a pet’s increased thirst.
Why urine specific gravity is important
Once clinical PU/PD has been confirmed, urine specific gravity (USG) is the next step in the diagnostic workup. It’s inexpensive, noninvasive, and easy to run in-house and yet can yield loads of valuable information. Because urine concentration fluctuates throughout the day, the urine sample for assessing USG should be obtained first thing in the morning before the pet has access to food or water.
“Beyond confirming the need for further workup, the USG can help narrow the differential list,” Kvitko-White said. Hyposthenuria (USG < 1.008) is most consistent with primary PD, diabetes insipidus (including all the causes of secondary nephrogenic diabetes insipidus), or hyperadrenocorticism (HAC), Kvitko-White added. “If you find a USG of less than 1.008, you’ve excluded both acute and chronic kidney disease (including secondary to leptospirosis or pyelonephritis). With 1 very simple, inexpensive test, you’ve begun to narrow down the list (of differentials).”
If urine is isosthenuric (USG 1.008-1.012), chronic kidney disease should be high on the list of differentials. However, many other diseases can produce isosthenuria, so chronic kidney disease can’t be confirmed based solely on this finding.
In dogs and cats whose urine is well concentrated, USG is higher than 1.030 and 1.035, respectively. This effectively rules out diabetes insipidus, Kvitko-White noted. HAC is also very unlikely.
What about other diagnostics?
The minimum database for a pet with PU/PD should include a complete blood count, chemistry panel, urinalysis, and total thyroxine (T4, which is more important in cats > 7 years old). Several important differentials such as hyperthyroidism, polycythemia, and diabetes mellitus can be ruled out effectively if this minimum database is normal. HAC is unlikely if all the liver enzymes are normal and there is no thrombocytosis. A dog with HAC should also have physical exam findings that support the diagnosis.
Once the minimum database is completed, additional diagnostic testing becomes more specific to investigate diseases such as HAC, hypoadrenocorticism, and leptospirosis.
Blood pressure should be checked to rule out hypertension. Additional laboratory studies include symmetric dimethylarginine(SDMA), which can point to a diagnosis of chronic kidney disease before azotemia, urine culture if pyelonephritis remains a reasonable differential, serum bile acids assay (if signs, history, and exam findings support liver disease or portosystemic shunt), and leptospirosis microscopic agglutination testing or urine polymerase chain reaction (if leptospirosis is still on the list based on history, clinical signs, and exam findings).
Imaging studies, such as survey radiography (abdominal and thoracic) and abdominal ultrasound, are also informative at this stage of the diagnostic workup, or earlier if the condition warrants.
What else could it be?
If all the previous diagnostic tests are unremarkable, psychogenic PD and diabetes insipidus remain on the list of possibilities. But clinicians need to differentiate these 2 conditions.
Psychogenic PD is more common in young, hyperactive dogs prone to high stress/anxiety. Owners may also report that the PU/PD is often intermittent, situational, or worse at certain times of the day or night. Oftentimes, these owners have already started restricting water during certain times of the day and night without ill effect to the pet.
Central diabetes insipidus (CDI) is often idiopathic and can occur in dogs of any age. Acquired CDI can occur with conditions such as head trauma or neoplasia affecting the brain (particularly the pituitary gland or hypothalamus). Primary nephrogenic diabetes insipidus is extremely rare and caused by a genetic defect.
Both psychogenic PD and diabetes insipidus cause hyposthenuria, but in CDI the clinical signs tend to be severe. Also, if water is restricted, a dog with psychogenic PD can eventually form concentrated urine (SG > 1.035), whereas the urine of a dog with CDI will never concentrate without medical intervention. It is important to remember that water restriction must be gradual due to washout of the medullary concentration gradient in psychogenic PD. Even a single concentrated urine sample confirms the diagnosis of psychogenic PD. Careful water deprivation testing that fails to concentrate can support a diagnosis of CDI, which is confirmed through a desmopressin trial.
In summary, obtaining a careful medical history and having a general understanding of the 5 mechanisms that initiate PU (and subsequently trigger PD) can help clinicians select the appropriate tests to confirm the patient’s diagnosis.
Karen Todd-Jenkins, VMD, is a medical writer and has remained in clinical practice for over 20 years. She is a member of the American Medical Writers Association and One Health Initiative.
