A quick review of canine exocrine pancreatic insufficiency
The pancreas has two separate functions within the body, often referred to as the exocrine and endocrine pancreata.
The pancreas has two separate functions within the body, often referred to as the exocrine and endocrine pancreata. The endocrine pancreas secretes hormones, including insulin and glucagon, which regulate blood glucose metabolism. The exocrine pancreas secretes zymogens and active enzymes that, ultimately, aid in digestion. Exocrine pancreatic insufficiency (EPI) is a condition of maldigestion and usually doe not involve the endocrine pancreas. In this article, we review the etiologic factors, diagnostic tools, and management recommendations for dogs with EPI.
NORMAL EXOCRINE PANCREATIC FUNCTION
The exocrine pancreas secretes many different zymogens for digesting carbohydrates, fats, and proteins (Figure 1). Protein digestion is catalyzed by the enzymes trypsin, chymotrypsin, and carboxypeptidase. These proteolytic digestive enzymes are initially released from the pancreas as the zymogens trypsinogen, chymotrypsinogen, and procarboxypeptidase, respectively, and are activated once they reach the small intestine. In the presence of chyme, enterocytes release enteropeptidase, which activates some of the trypsinogen. Additionally, the newly formed trypsin assists in activating all three zymogens. This delayed activation prevents autodigestion of pancreatic proteins. Once activated, trypsin and chymotrypsin break down proteins into smaller peptides, while carboxypeptidase further processes some of these peptides into amino acids.
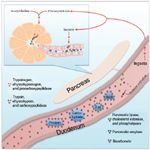
Figure 1. Normal exocrine pancreatic function in animals.
Carbohydrate digestion is facilitated by pancreatic amylase secretion, which hydrolyzes most carbohydrates into disaccharides and some trisaccharides. Finally, the exocrine pancreas facilitates fat digestion by releasing the enzymes pancreatic lipase, cholesterol esterase, and phospholipase and the zymogen procolipase, which is activated by trypsin to form colipase.
Exocrine pancreatic secretions contain digestive enzymes and sodium bicarbonate in an aqueous solution. Acetylcholine and cholecystokinin stimulate the release of digestive enzymes, while secretin stimulates the release of large quantities of bicarbonate and water. The digestive enzymes are produced and secreted by the pancreatic acinar cells, while epithelial cells of the pancreatic ductules secrete bicarbonate. Once within the duodenum, sodium bicarbonate neutralizes gastric secretions.1
EXOCRINE PANCREATIC INSUFFICIENCY
In patients with EPI, inadequate production of digestive enzymes by the pancreatic acinar cells leads to maldigestion and malabsorption of nutrients. The persistence of undigested food within the small intestine often results in bacterial overgrowth, further compromising intestinal function. Fortunately, the pancreas has a high reserve capacity, so signs of maldigestion do not occur until 90% of the exocrine pancreatic function is lost.2 In rare cases of EPI in people, there is selective deficiency of individual pancreatic enzymes. Isolated lipase deficiency has been described in a single dog.3
CAUSES OF EPI
Causes of EPI include pancreatic acinar atrophy, chronic pancreatitis, pancreatic hypoplasia, and neoplasia.
Pancreatic acinar atrophy
The most common cause of EPI in dogs is pancreatic acinar atrophy. The severity of this condition ranges from subclinical disease to a complete absence of secretory capacity.2 Pancreatic acinar atrophy is thought to be an immune-mediated condition that begins with lymphocytic pancreatitis.4 Selective destruction of acinar cells with replacement by atypical parenchyma, ductal structures, and adipose tissue is seen in the late stages of the disease.
Immunohistochemical analysis of pancreatic biopsy samples from dogs with subclinical EPI reveals a predominance of intra-acinar CD4+ and CD8+ T lymphocytes, supporting an immune-mediated cause.5 Other histologic characteristics include piecemeal tissue destruction, large groups of lymphocytes that resemble lymphoid follicle germinal centers, and necrotic and apoptotic acinar cell death.4
Chronic pancreatitis
In people, most cases of EPI are secondary to chronic pancreatitis,1 but the prevalence of cases of EPI that develop secondary to chronic pancreatitis in dogs is still unclear.2 Pancreatic fibrosis can affect the islet cells of the endocrine pancreas and the acinar cells of the exocrine pancreas.2 In one study, two of four dogs with EPI resulting from chronic pancreatitis also had diabetes mellitus.6 In people and dogs with EPI resulting from chronic pancreatitis, diabetes mellitus usually precedes the development of EPI.6
Pancreatic hypoplasia
A potential cause of EPI is congenital hypoplasia of the pancreas. This cause has been proposed in puppies in particular, although published data lack histologic evidence to support this theory.2,7
Neoplasia
Rarely, pancreatic neoplasia can occlude the pancreatic duct, prohibiting release of pancreatic enzymes into the duodenum. Although the ability of the pancreatic acinar cells to produce pancreatic enzymes may not be affected, clinical signs of EPI may result.2
DIAGNOSING EPI
Signalment
EPI can affect any breed, but German shepherds may be overrepresented. The two breeds most commonly affected by pancreatic acinar atrophy, German shepherds and rough-coated collies, are thought to have an autosomal recessive inherited form of the disease.8,9 Other breeds overrepresented among dogs with EPI due to any cause include Cavalier King Charles spaniels, chow chows, and English setters.7,10 The common causes associated with each commonly reported breed are listed in Table 1.

Table 1 Most Common Signalment and Causes of EPI in Dogs*
In a recent case report, pancreatic atrophy was described for the first time in several racing greyhound puppies. It is unclear whether environmental factors contributed to the development of EPI in these dogs or whether a true breed predisposition to this condition exists.11
The median age of dogs with EPI is variable, depending on the cause (Table 1).
Clinical signs
Dogs with EPI present with signs of maldigestion, primarily weight loss despite an increased appetite and diarrhea or loosely formed feces. Feces are usually yellow or gray, are increased in volume, and may appear undigested or pulpy. In most cases, fecal consistency is loosely formed, but dogs may experience severe watery diarrhea initially. The diarrhea is usually accompanied by steatorrhea, flatulence, and borborygmi. Some dogs with EPI also experience vomiting. Along with the weight loss, these dogs may have a poor coat. They may also seem nervous, aggressive, or irritable as a result of abdominal discomfort.2,12,13
Clinical pathology
EPI rarely affects serum chemistry profile and complete blood count results. Amylase and lipase activities are not useful in diagnosing EPI. Occasionally, a serum chemistry profile may reveal hypocholesterolemia due to fat maldigestion. Elevation of alanine transaminase (ALT) activity has been documented uncommonly, although the cause of this is unknown. One theory is that an increased uptake of hepatotoxins occurs through the compromised small intestinal wall.2
Diagnostic imaging
Dogs with marked weight loss and loss of intra-abdominal fat secondary to EPI may have decreased serosal detail on abdominal radiographs. No specific ultrasonographic abnormalities have been reported in dogs with EPI.14
Histologic examination
Pancreatic biopsies are rarely necessary for diagnosing EPI, and the results may be misleading if acinar atrophy, fibrosis, or hypoplasia is not diffuse.2 Pathologic findings within the pancreas may help you determine the cause of EPI, but a diagnosis of EPI must be based on findings from tests of pancreatic function rather than histologic appearance.
Specific diagnostic tests
A diagnosis of EPI is most reliably based on clinical signs and pancreatic function test results. The gold standard for diagnosing EPI in people is in vitro analysis of pancreatic secretions obtained from the duodenum following stimulation by cholecystokinin and secretin.15 This technique has proved to be difficult and impractical in dogs.16 The recommended test for diagnosing EPI in dogs is the canine serum trypsin-like immunoreactivity (cTLI) by radioimmunoassay.
Canine serum trypsin-like immunoreactivity. This test measures the trypsinogen that has entered the bloodstream directly from the pancreas. Enzymes that originated or were activated within the intestinal lumen are not measured, which eliminates any interference by intestinal inflammation.2 The test is also not affected by exogenous sources of pancreatic enzymes because it is species-specific.16 The reference range for cTLI is 5.7 to 45.2 µg/L, with values below 2.5 µg/L being highly diagnostic for EPI when concurrent clinical signs exist.2
Although not affected by intestinal inflammation, the cTLI test may be affected by pancreatic inflammation.6,17 Pancreatitis affecting any residual functioning acinar cells may falsely elevate cTLI concentrations.17 Dogs with EPI may have normal cTLI concentrations in the presence of kidney disease because trypsinogen is renally excreted.2,18 The cTLI concentrations could be normal in animals with EPI secondary to pancreatic duct obstruction.16 Postprandial samples may also result in falsely elevated cTLI results.2,18 Thus, the sample should be obtained in a patient that has been fasted for 12 to 18 hours, and the sample should be nonhemolyzed. Despite these potential interferences, the cTLI test is considered 100% sensitive and specific for EPI with results below 1.9 µg/L.16
Canine TLI testing by radioimmunoassay is available through commercial laboratories such as the Texas A&M University College of Veterinary Medicine's Gastrointestinal Laboratory, Antech Diagnostic Laboratory, or Idexx Laboratories. An in-house ELISA test kit to detect cTLI has been investigated.19
Serum pancreatic lipase immunoreactivity. Serum pancreatic lipase immunoreactivity (PLI), used to detect pancreatitis, is also a sensitive test for diagnosing EPI. Unfortunately, poor specificity makes it inferior to the cTLI test for this purpose.20 The newest version of the PLI test, the Spec cPL Test (Idexx Laboratories), has been optimized for diagnosing pancreatitis but may not be useful for diagnosing EPI. In general, all available forms of pancreatic lipase testing should be used for diagnosing pancreatitis rather than pancreatic insufficiency.
Fecal pancreatic elastase 1. Fecal pancreatic elastase 1 is a zymogen produced exclusively within pancreatic acinar cells.21 It survives intestinal transit unchanged and is unaffected by intestinal inflammation.21 An ELISA test for fecal pancreatic elastase 1 (Elastase 1 Canine—ScheBo Biotech AG) in dogs is available and is marketed as a screening tool for EPI. A fecal pancreatic elastase 1 value < 10 µg/g feces indicates a diagnosis of EPI. Values > 40 µg/g feces indicate normal exocrine pancreatic function, while values between 10 and 40 µg/g feces are borderline results. In borderline cases, further testing is recommended.22 This test is species-specific, so it is unaffected by pancreatic enzyme supplementation. Fecal pancreatic elastase 1 measurement has been used extensively in diagnosing EPI in people.21
Other tests. Several tests for EPI have been rendered nearly obsolete by the advent of the cTLI test. These include tests for fecal proteolytic activity, which should be low in dogs with EPI, including the x-ray film method, the azocasein method, and the radial enzyme diffusion method.2,16 The bentiromide test, also known as the serum n-benzoyl-L-tyrosine-p-aminobenzoic acid test (BT-PABA), is an indirect method of assessing the presence of chymotrypsin in the blood.16-18,23
Other tests that are no longer actively used include the starch tolerance test, the D-xylose absorption test, and the oral fat absorption test.21 None of these tests are as sensitive and specific as the cTLI test for diagnosing EPI.
SUBCLINICAL EPI
As previously discussed, clinical EPI does not result until 90% of the secretory capacity of the exocrine pancreas is lost. Mild decreases in pancreatic function have been detected in clinically normal dogs. This condition has been termed subclinical EPI and may reflect partial pancreatic acinar atrophy. In affected dogs, repeated cTLI results fall between 2.5 and 5 µg/L; a single subnormal value is not diagnostic for subclinical EPI.2 In one study, 20 of 35 (57%) of dogs with subnormal results had normal cTLI concentrations when retested.23 Further, the detection of reduced pancreatic function should not be mistaken for progressive disease. The length of the subclinical phase is highly variable, and some dogs never develop clinical EPI.24
Dogs with borderline fasting cTLI results can be further tested by using the TLI stimulation test, in which cTLI is measured before and after pancreatic stimulation.23 Food is usually used for pancreatic stimulation. Endogenous stimulation by intravenous administration of cholecystokinin and secretin has also been effective experimentally in one study.23 A diagnosis of EPI is confirmed if there is no response to stimulation. Dogs with subclinical EPI typically have low fasting cTLI results but normal post-stimulation results.23,24
TREATMENT
Dogs with subclinical EPI do not require treatment. In cases of suspected pancreatic acinar atrophy, the efficacy of immunosuppressive therapy has been studied in hopes of slowing or preventing the development of clinical EPI. Because of the unpredictable progression of subclinical EPI, the use of immunosuppressive medications is not recommended.2,24
Pancreatic enzyme replacement
The mainstay of treatment in dogs with clinical EPI of any cause is pancreatic enzyme replacement. Commercially available preparations are generally derived from porcine pancreas and contain lipase, amylase, and protease for digestion of fats, carbohydrates, and proteins, respectively. The veterinary products come in powdered and uncoated tablet forms (e.g. Viokase-V—Fort Dodge Animal Health; Pancrezyme—Virbac Animal Health), and products approved for use in people are available in all forms and can be used instead if needed. Alternatively, chopped, raw bovine or porcine pancreas can be fed directly.
Raw and powdered forms of pancreatic enzyme supplementation have the greatest efficacy, although uncoated tablets may be crushed, making them as efficacious as the powdered form.25 Enteric-coated pancreatic enzyme tablets are not recommended. Theoretically, they protect the enzymes from gastric acidity, but the enteric coating requires pancreatic bicarbonate for removing the coating, making this form inappropriate for dogs with EPI. Supplementing bicarbonate is not recommended, as it increases the production of gastric acid.2,25,26

Table 2 Doses for Pancreatic Enzyme Replacement Therapy
Pancreatic enzyme supplementation doses are listed in Table 2. Patients that respond poorly to supplementation may improve with dose increases, alternate forms of enzyme replacement, incubation of food with enzymes for 20 to 30 minutes before feeding, or concurrent administration of H2 blockers.2 Theoretically, H2 blockers such as cimetidine or famotidine would reduce gastric acidity and better preserve the enzymes for intestinal use.12 Administration of these drugs could begin during initiation of treatment in all dogs or may be reserved for those with suboptimal response to treatment. Given the cost of treatment, pancreatic enzyme dose reduction can be attempted. Some dogs have been managed adequately with a 50% dose reduction.13
Pancreatic enzyme supplementation may cause gastrointestinal distress (including diarrhea, cramping, and nausea) at high doses.27 Additionally, oral ulceration and bleeding have been reported, presumably the result of digestive enzymes contacting the oral mucosa. Ulcerations may be alleviated by reducing the dose28,29 or by incubating the enzymes in the food for longer periods.28
Alternative sources of pancreatic enzymes have been investigated. Fungal lipase, derived from Aspergillus species, is acid-resistant, so it survives gastric transit but, unfortunately, is deactivated by proteases and bile acids within the small intestine, making it inferior to traditional supplementation.30,31 Bacterial lipase, derived from Burkholderia plantarii, survives gastric and intestinal transit. It has been proved to correct steatorrhea more effectively than porcine pancreatic enzyme supplements and requires much lower doses.30,32 Bacterial-derived enzymes may be ideal for treating EPI, but high cost and reduced availability may limit their use.
Dietary modification
Dietary modification may improve clinical signs in dogs with EPI. In most cases, trial and error is needed to discern the appropriate diet for a patient.33 In people with EPI, high-fat diets are used to maximize weight gain.34 This type of diet may be helpful in dogs, but it can worsen diarrhea and may increase the requirement for enzyme supplementation.33 In most cases, a moderate-fat diet is recommended.2 Low-fat diets have not been shown to improve clinical signs over moderate-fat diets.13
Replacing diets rich in long-chain fatty acids with those containing more medium-chain fatty acids, such as Purina Veterinary Diet EN (Nestlé Purina), also does not seem to improve clinical signs, although this may increase intestinal absorption of cholesterol and fat-soluble vitamins, which can be decreased in patients with EPI.35 High-fiber diets are generally not recommended because they increase fecal mass, are poorly digestible, and can inhibit the absorption of other nutrients.33 However, some dogs can show improvement in fecal firmness and a decrease in flatulence and borborygmi when receiving high-fiber diets.33 Finally, hydrolyzed protein diets have resulted in improved body condition in some dogs with EPI.36
We typically recommend initiating therapy with an easily digestible diet such as Purina Veterinary Diet EN or Iams Veterinary Formula Intestinal—Low-Residue (Iams). If no response is seen, the dosage of pancreatic enzyme supplementation is usually adjusted before alternative diets are used. In general, treats are not recommended since it is impractical to administer enzyme supplementation with each treat to aid in digestion. If clients insist upon giving treats, we recommend waiting until signs of EPI are well-controlled.
PATIENT FOLLOW-UP
Dogs with EPI should be evaluated regularly. After new treatments are initiated or altered, evaluate patients every two to four weeks for weight gain and improved body condition. The frequency of evaluation depends on the severity of clinical signs. Instruct owners to carefully monitor fecal consistency and volume and report any changes. After initial stabilization, patients with excellent response to therapy may be examined annually or biannually as recommended for healthy adult dogs. Follow-up monitoring of cTLI concentrations is not indicated since enzyme supplementation and other treatments are not expected to improve the secretory capacity of the exocrine pancreas.
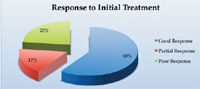
Figure 2. Percentage of dogs responding to pancreatic enzyme supplementation at recommended dosages. Source: Reference 9.
PROGNOSIS
Dogs with EPI require lifelong treatment. Improvement generally occurs within the first few weeks and stabilizes thereafter.2 Figure 2 shows the response to initial treatment, and Figure 3 shows survival data. About 20% of dogs are euthanized as a direct result of their EPI, usually because of the cost of treatment or lack of clinical improvement.2 Dogs that respond positively to treatment may experience short relapses, but, fortunately, permanent deterioration rarely occurs.25
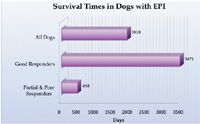
Figure 3. Mean survival time for dogs with EPI based on initial response to therapy. Source: Reference 9.
CONCLUSION
EPI should be included in the differential list for any dog with small bowel diarrhea. With the advent of more sensitive and specific testing, EPI has become relatively simple for clinicians to diagnose. Treatment is generally rewarding but can be expensive and may present a management challenge in certain cases. Given the multitude of concurrent and secondary diseases that develop, it is also important to remain open to these possible conditions even after EPI has been diagnosed (see the sidebar titled "Canine EPI: Concurrent and secondary diseases").
Jessica A. Morgan, DVM
Lisa E. Moore, DVM, DACVIM
Affiliated Veterinary Specialists
9905 South U.S. Highway 17/92
Maitland, FL 32751
REFERENCES
1. Hall JE. Secretory function of the alimentary tract. In: Guyton & Hall's textbook of medical physiology. 11th ed. Philadelphia, Pa: Saunders, 2005;791-807.
2. Westermarck E, Wiberg M, Steiner J, et al. Exocrine pancreatic insufficiency in dogs and cats. In: Ettinger SJ, Feldman EC, eds. Textbook of veterinary internal medicine. 6th ed. St Louis, Mo: Elsevier Saunders, 2005;1492–1495.
3. Xenoulis PG, Fradkin JM, Rapp SW, et al. Suspected isolated pancreatic lipase deficiency in a dog. J Vet Intern Med 2007;21(5):1113-1116.
4. Wiberg ME, Saari SA, Westermarck E. Exocrine pancreatic atrophy in German shepherd dogs and rough-coated collies: an end result of lymphocytic pancreatitis. Vet Pathol 1999;36(6):530-541.
5. Wiberg ME, Saari SA, Westermarck E, et al. Cellular and humoral immune responses in atrophic lymphocytic pancreatitis in German shepherd dogs and rough-coated collies. Vet Immunol Immunopathol 2000;76(1-2):103-115.
6. Watson PJ. Exocrine pancreatic insufficiency as an end stage of pancreatitis in four dogs. J Small Anim Pract 2003;44(7):306-312.
7. Boari A, Williams DA, Famigli-Bergamini P. Observations on exocrine pancreatic insufficiency in a family of English setter dogs. J Small Anim Pract 1994;35(5):247–250.
8. Westermarck E, Pamilo P, Wiberg M. Pancreatic degenerative atrophy in the collie breed: a hereditary disease. Zentralbl Veterinarmed A 1989;36(7):549-554.
9. Moeller EM, Steiner JM, Clark LA, et al. Inheritance of pancreatic acinar atrophy in German shepherd dogs. Am J Vet Res 2002;63(10):1429-1434.
10. Batchelor DJ, Noble PJ, Cripps PJ, et al. Breed associations for canine exocrine pancreatic insufficiency. J Vet Intern Med 2007;21(2):207-214.
11. Brenner K, Harkin KR, Andrews GA, et al. Juvenile pancreatic atrophy in greyhounds: 12 cases (1995-2000). J Vet Intern Med 2009;23(1):67-71.
12. Batchelor DJ, Noble PJ, Taylor RH, et al. Prognostic factors in canine exocrine pancreatic insufficiency: prolonged survival is likely if clinical remission is achieved. J Vet Intern Med 2007;21(1):54-60.
13. Westermarck E, Junttila JT, Wiberg ME. Role of low dietary fat in the treatment of dogs with exocrine pancreatic insufficiency. Am J Vet Res 1995;56(5):600-605.
14. Hecht S, Henry G. Sonographic evaluation of the normal and abnormal pancreas. Clin Tech Small Anim Pract 2007;22(3):115-121.
15. Moolsintong P, Burton FR. Pancreatic function testing is best determined by the extended endoscopic collection technique. Pancreas 2008;37(4):418-421.
16. Williams DA, Batt RM. Sensitivity and specificity of radioimmunoassay of serum trypsin-like immunoreactivity for the diagnosis of canine exocrine pancreatic insufficiency. J Am Vet Med Assoc 1988;192(2):195-201.
17. Keller ET. High serum trypsin-like immunoreactivity secondary to pancreatitis in a dog with exocrine pancreatic insufficiency. J Am Vet Med Assoc 1990;196(4):623-626.
18. Williams DA. Exocrine pancreatic insufficiency. In: Kirk RW, Bonagura JD. Current veterinary therapy X small animal practice. Philadelphia, Pa: Saunders, 1989;927-932.
19. Waritani T, Okuno Y, Ashida Y, et al. Development of a canine trypsin-like immunoreactivity assay system using monoclonal antibodies. Vet Immunol Immunopathol 2002;87(1-2):41-49.
20. Steiner JM, Rutz GM, Williams DA. Serum lipase activities and pancreatic lipase immunoreactivity concentrations in dogs with exocrine pancreatic insufficiency. Am J Vet Res 2006;67(1):84-87.
21. Spillmann T, Wittker A, Teigelkamp S, et al. An immunoassay for canine pancreatic elastase 1 as an indicator for exocrine pancreatic insufficiency in dogs. J Vet Diagn Invest 2001;13(6):468-474.
22. ScheBo-Biotech AG Elastase 1 canine test page. Available at: www.schebo.com/index.php?p_id=2664&p=13. Accessed Aug 21, 2009.
23. Wiberg ME, Nurmi AK, Westermarck E. Serum trypsinlike immunoreactivity measurement for the diagnosis of subclinical exocrine pancreatic insufficiency. J Vet Intern Med 1999;13(5):426-432.
24. Wiberg ME, Westermarck E. Subclinical exocrine pancreatic insufficiency in dogs. J Am Vet Med Assoc 2002;220(8):1183-1187.
25. Pidgeon G, Strombeck DR. Evaluation of treatment for pancreatic exocrine insufficiency in dogs with ligated pancreatic ducts. Am J Vet Res 1982;43(3):461-464.
26. Wiberg ME, Lautala HM, Westermarck E. Response to long-term enzyme replacement treatment in dogs with exocrine pancreatic insufficiency. J Am Vet Med Assoc 1998;213(1):86-90.
27. Plumb DC. Plumb's veterinary drug handbook. 5th ed. Ames, Iowa: Wiley-Blackwell, 2005;855-856.
28. Snead E. Oral ulceration and bleeding associated with pancreatic enzyme supplementation in a German shepherd with pancreatic acinar atrophy. Can Vet J 2006;47(6):579-582.
29. Rutz GM, Steiner JM, Williams DA. Oral bleeding associated with pancreatic enzyme supplementation in three dogs with exocrine pancreatic insufficiency. J Am Vet Med Assoc 2002;221(12):1716-1718, 1714.
30. Layer P, Keller J. Lipase supplementation therapy: standards, alternatives, and perspectives. Pancreas 2003;26(1):1-7.
31. Griffin SM, Alderson D, Farndon JR. Acid resistant lipase as replacement therapy in chronic pancreatic exocrine insufficiency: a study in dogs. Gut 1989;30(7):1012-1015.
32. Suzuki A, Mizumoto A, Sarr MG, et al. Bacterial lipase and high-fat diets in canine exocrine pancreatic insufficiency: a new therapy of steatorrhea? Gastroenterology 1997;112(6):2048-2055.
33. Westermarck E, Wiberg ME. Effects of diet on clinical signs of exocrine pancreatic insufficiency in dogs. J Am Vet Med Assoc 2006;228(2):225-229.
34. Keller J, Layer P. Pancreatic enzyme supplementation therapy. Curr Treat Options Gastroenterol 2003;6(5):369–374.
35. Rutz GM, Steiner JM, Bauer JE, et al. Effects of exchange of dietary medium chain triglycerides for long-chain triglycerides on serum biochemical variables and subjectively assessed well-being of dogs with exocrine pancreatic insufficiency. Am J Vet Res 2004;65(9):1293-1302.
36. Biourge VC, Fontaine J. Exocrine pancreatic insufficiency and adverse reaction to food in dogs: a positive response to a high-fat, soy isolate hydrolysate-based diet. J Nutr 2004;134(8 Suppl):2166S-2168S.